The Role Of Botox In The Management Of Tmj Disorders – A Systematic Review
Fahad Amer Almutairi 1, Moath Mansour Almansour 1, Ibraheem Mansour Almansour 2, Riyad Abdullah Alwazzan 3, Hisham Mohammed Alrashaid 4, Mohammad Abdul Baseer 5*
1 Dental Intern, Majmaah University, Saudi Arabia.
2 Dental student, Majmaah University, Saudi Arabia.
3 General Dental Practitioner, Riyadh, Saudi Arabia.
4 General Dental Practitioner, Ministry of Health, Riyadh, Saudi Arabia.
5 Department of Preventive Dentistry, College of Dentistry, Riyadh Elm University, Riyadh, Saudi Arabia.
ABSTRACT
Introduction: The majority of people who suffer from temporomandibular disorders (TMD) opt for medical help when they encounter severe chronic pain. With a change in treatment modality, it is seen that botulinum toxin (BTX) has gained much popularity. Therefore, this systematic review aimed to assess the current status of BTX in the management of TMD and how effective it is to treat the pain and other associated symptoms seen in patients affected with TMD.
Material and Methods: A detailed electronic literature search was conducted, including specified inclusion criteria to identify all the relevant high evidence-based randomized controlled trials (RCTs). Based on the inclusion and exclusion criteria, they then assessed for bias using a framework outlined in the Cochrane Handbook.
Results: After thorough selection criteria, 5 Randomized Controlled Trials (RCTs) were included in the study. The review's primary focus was assessing pain level by using different diagnostic tools to qualitatively observe the effectiveness of botulinum toxin in the management of temporomandibular myofascial pain. The secondary outcomes included measurement of changes in mouth opening, psychological status, day-to-day functional and social activities, and muscle hyperactivity.
Conclusion: The results from the included studies in the review support the benefits of BTX in reducing the symptoms associated with TMD, but further research is required to show the effectiveness of BTX. To achieve a clear understanding of the benefits of BTX in TMD, RCT with a large sample size, homogeneous study design, longer follow-up period, and based on a standardized diagnostic method must be performed.
Key words: temporomandibular disorders, botulinum toxin, myofascial pain, randomized controlled trials, a systematic review.
Introduction
Temporomandibular disorders (TMD) are the commonly encountered problems affecting TMJ and its associated structures. The American Association for Dental Research (AADR) has defined these disorders as "a group that involves musculoskeletal and neuromuscular conditions where apart from the TMJ, the muscles of mastication and other surrounding structures are also involved".1-3 It is known to cause a severe amount of pain in the orofacial region, such that it is the third most common factor after headache and backache.4-6 TMD consists of different kinds of disorders, and there is numerous classification used for its categorization.
Majority of people who suffer from temporomandibular disorders (TMD) opt for medical help when they encounter severe chronic pain. The pain can be in the form of headaches or facial pain.7, 8 The symptoms of the pain can give the illusion of migrainous headaches. It has been a constant challenge for doctors to diagnose and treat this type of myofascial pain. This pain does not occur in one particular area and encompasses the masticatory muscles' spasm and leads to functional limitations. It is reported that more than half of the patients who are diagnosed with TMD suffer from temporomandibular myofascial pain.9 It affects 5% to 10% of the population, with the majority being the females. This myofascial pain, which occurs in TMD, is a subtype of fibromyalgia. It generally activates when there are painful trigger points that spike up due to parafunctional habits, inadequate postures, and any abnormal variation in the social, physical, mental, and behavioral characteristics.10, 11 Myofascial pain is not area-specific and may radiate to other areas also beyond the primary site. It is often accompanied by muscle fatigue, reduced joint movements 12, and headaches. 13
TMD commonly affects adults, but few cases have also been diagnosed in childhood.7, 8 TMD's etiology is not specific as it is caused due to a combination of a few other factors that predispose an individual to such disorders. The various elements may be related to unfavorable occlusion, stress, or postural defects. All these alterations result in long term tension on the joint and associated structures as it is a prevalent issue such that it has become a global health issue that demands adequate attention. 14
Proper management of pain related to TMD involves different factors and focuses on presenting signs and symptoms. There can be either involvement of a single muscle or multiple muscles, depending on the symptoms' presentation. A broad scope of therapeutic options is currently available. It includes tricyclic antidepressants and other drugs, exercise, posture correction, trigger point injections, biofeedback, transcutaneous electrical nerve stimulation (TENS), and muscle relaxants.15 Depending on the disease severity, the treatment should be planned.16
Over the past few decades, there has been a shift in the management of TMD's treatment modality. Initially, the main focus was to conduct invasive dental procedures or surgery; however, presently, the attention is on managing psychosocial/social factors.17, 18 Due to bruxism, the dental treatment involved stabilization with a splint to manage the TMD. However, all these provide symptomatic relief. Therefore, currently, botulinum toxin (BTX) has gained much popularity and is used to treat TMD.
Botulinum toxin (BTX), synthesized by Gram-positive anaerobe Clostridium botulinum, is a potent neurotoxin. It causes muscle relaxation as it temporarily blocks the release of acetylcholine from presynaptic cholinergic nerve terminals. When there is the formation of new synaptic connections 19, its effect subsides, and therefore, there is the restoration of original nerve terminals.20, 21 Additionally, it blocks the release of inflammatory mediators, including glutamate and substance P. 22, 23 BTX was initially used for the treatment of neuromuscular disorders.24 However, its clinical usage has expanded to different fields, including the pain disorders management of musculoskeletal origins, especially in myofascial pain.25 This is due to its property to act as a muscle relaxant and demonstration of pain-relieving effects. Recently, few studies have reported the important therapeutic role of BTX in diminishing myofascial pain and other associated problems seen in TMJ disorders.26, 27
However, significant findings related to its utility in TMD are still lacking. Hence, this systematic review aims to evaluate the current evidence to assess the therapeutic efficacy of BTX in the management of TMD.
Materials and Methods
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [PRISMA Checklist 2009] the present review was reported. This study was registered with the research center of Riyadh Elm University (FIRP/2020/82).
Research Question
The clinical question to be answered in this systematic review was framed in terms of a PICO question {problem (P), intervention (I), comparison (C), and outcome (O)} as follows:
In patients who were diagnosed with TMD (P), does the treatment with BTX injection (I), or any other alternative medicine (C) or placebo, results in improvement of pain and other associated signs and symptoms (O).
Literature search
A computerized literature search was performed in MEDLINE (PubMed), the Cochrane Library, EBSCO host (Dentistry & Oral Sciences Source), and Embase databases from inception until 10th July 2020. The Cochrane Handbook for Systematic Reviews of Interventions was used to include relevant controlled trials. References for textbooks and selected articles were screened to identify any related studies. The literature search was performed by adding the following terms related to botulinum toxin: 'Botulinum toxin' [MeSH] or 'Botox' or 'Botulinum,' "Botulinum Toxins, Type A." or "Injections" [MeSH] along with "Temporomandibular Joint Disorder" [MeSH] or "Temporomandibular Joint Dysfunction" [MeSH] or 'Temporomandibular pain dysfunction syndrome' or "Myofascial Pain" or "Myofascial Pain Syndrome" or "Myofascial Pain Dysfunction Syndrome" or 'Facial Pain' [MeSH] or "Temporomandibular Joint Dysfunction Syndrome."
Eligibility criteria for the studies
Inclusion Criteria
The relevant studies' full-text articles were then obtained and reviewed by the reviewers independently to ensure that the studies met the inclusion criteria. The inclusion criteria were as follows:
Exclusion criteria were as follows:
Study selection
The initiating search strategy selected the relevant studies in two stages. Firstly, the authors were independently involved with the process of this study and extracted the necessary information. All available titles and abstracts were identified and scanned by them, such that the included studies met the inclusion criteria. In the second stage, full-text articles were thoroughly investigated by both the reviewers independently. Papers that had cited these articles were also identified through the Science Citation Index (http://www.isinet.com) to identify potentially relevant articles' subsequent primary research. The disagreements were mutually discussed, and a consensus was made before the inclusion of the study in this review.
Data extraction and Quality Assessment
Studies that fulfilled the inclusion criteria were processed for data extraction. The review's primary focus was the assessment of pain level by using different diagnostic tools to qualitatively observe the effectiveness of botulinum toxin in the management of temporomandibular myofascial pain. The secondary outcomes included measurement of changes in the following: mouth opening, psychological status, and day to day functional and social activities, and muscle hyperactivity.
The reviewers independently extracted the data. It included the data on study characteristics - the name of the first author, year of publication, the total number of participants and dropouts, pre-treatment status, diagnostic criteria and muscles involved, assessment of pain, study design, follow-up period, and method of injecting Botox. The included studies were submitted to quality assessment according to the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions 5.0.0.28
Risk of bias evaluation
The risk of bias for the selected studies was evaluated with the Cochrane risk of bias tool. 29 After a thorough assessment, the reviewers independently categorized the selected studies as "high risk of bias," or "low risk of bias," or "unclear risk of bias" based on the following parameters: concealment of allocation sequence, randomization methods, study design, blinding of study subjects and personnel, any missing outcome data, measurement of the intended outcome.
As there was heterogeneity associated with the methodology and assessment in the included studies; therefore, we could not perform a meta-analysis.
Results
Search and Study selection
The process of retrieving and screening the studies included in this systematic review is shown in Figure 1. After an initial search, a total of 537 articles were identified. After screening the titles and abstracts, only 205 were found to be relevant. The remaining 332 studies were excluded, as some of them were duplicated, irrelevant, and others did not justify the inclusion criteria. Then, 53 studies, which were full-text articles, were critically reviewed by reviewers independently for eligibility. Finally, five high-quality randomized controlled trials that met all the inclusion criteria were included in the review.
Characteristics of Studies Included in the Review
The five studies included were assessed for the risk of bias, as shown in Table 1. The Cochrane risk tool was evaluated on various parameters and showed a low risk of bias. Therefore, we focused on high evidence-based RCT, where the scope of any random error due to bias was minimal. In these studies, the author, sample size, diagnosis, assessment of primary and secondary outcome, a tool used for evaluating the issue, the follow-up period, and results obtained concerning treatment and control group are shown in Table 2.
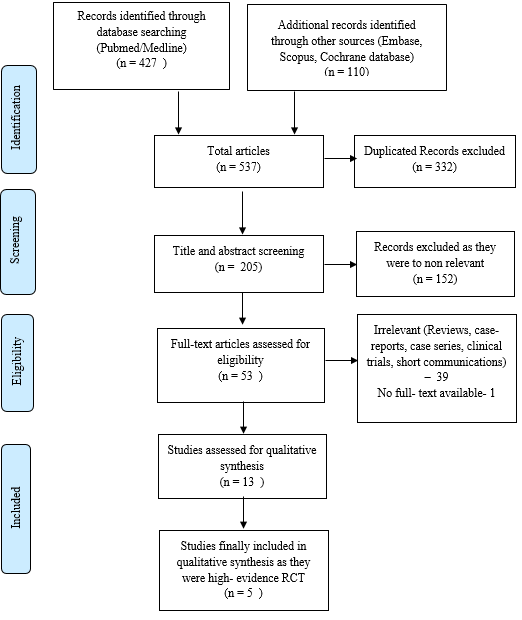
Figure 1. Flow diagram illustrating the literature search and selection criteria (according to PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis) 30
|
Included studies |
Random sequence generation |
Allocation concealment |
Blinding of participants and personnel |
Blinding of outcome assessment |
Incomplete outcome data |
Selective reporting |
Other bias |
|
Kurtoglu et al. 31 |
Risk - low |
Risk -unclear |
Risk - low |
Risk- low |
Risk- low |
Risk- low |
Risk – unclear |
|
Ernberg et al. 32 |
Risk- low |
Risk- low |
Risk- low |
Risk – unclear |
Risk - high |
Risk- low |
Risk- low |
|
Carli et al. 33 |
Risk- low |
Risk- low |
Risk-high |
Risk- low |
Risk-high |
Risk-unclear |
Risk- unclear |
|
Nixdorf et al. 34 |
Risk- low |
Risk- low |
Risk- low |
Risk- unclear |
Risk-high |
Risk- low |
Risk- unclear |
|
Patel et al. 35 |
Risk- low |
Risk- low |
Risk-unclear |
Risk-unclear |
Risk-high |
Risk- low |
Risk-low |
Table 1. The risk of bias for the included papers according to the Cochrane Handbook guidelines.
|
Author (Year) |
Sample size (Test/control) |
Test/Control |
Diagnosis |
Primary outcome measure |
Results summary |
Secondary outcome measure |
Results summary |
|
Kurtoglu et al. 31 |
12/12 |
T = BTX, C = saline |
TMD (myofascial pain) |
Assessment of pain based on biobehavioral questionnaire relating to RDC/TMD |
Pain At Baseline: C = 58.9 T = 56.1 14 days: C = 51.1 T = 45.8 28 days: C = 51.4 T = 43.9 |
EMG readings of the anterior temporal and masseters muscles when at rest and during maximal clenching |
EMG Readings
Mean EMG: Baseline: C = 200.0/529.3 T = 206.3/296.0 14 days: C = 252.3/498.8 T = 165.0/199.0 28 days: C = 212.8/540.8 T = 221.0/256.0 |
|
Ernberg et al. 32 |
21/21 |
T = BTX C = isotonic saline |
TMD (myofascial pain) |
The pain was assessed by - VAS (0–100 mm) - Graded Chronic Pain Scale was used to calculate the Pain Intensity |
Pain Mean results, baseline (Pain intensity, VAS): C = 54 T = 58 1 month: C = 27, T = 35 (wrt baseline) 3 months: C = 24 T = 34 (wrt baseline) |
Maximum mouth opening, Physical functioning, Emotional functioning, Global improvement and adverse event |
Maximum mouth opening (mm) Mean results, baseline: C = 43.4 T = 42.7 1 month: C = 44.3, T = 44.3 (wrt baseline) 3 months: C = 44.2 T = 44.3 (wrt baseline).
No change is seen wrt to Physical functioning, emotional functioning, global improvement between the control group and treatment group. |
|
Carli et al. 33 |
7/8 (this does not include the three dropouts) |
T = BTX C = low level laser |
Myofascial pain |
Pain (VAS) |
Pain Baseline: C = 7 T = 7 Day 30: C = 3.5, T = 3 |
Mouth opening |
Maximum mouth opening
Baseline: C = 42, T = 38 Day 30 = C = 42, T = 36 |
|
Nixdorf et al. 34 |
15/15 |
T = BTX, C = saline |
TMD (RDC/TMD – group I.a. and II.b. or myofascial pain without and with limited mouth opening) |
Pain VAS (0–100 mm) |
Baseline and after 8 weeks: mean difference in VAS 1) Pain intensity C = 1 mm reduction, T = 19 mm reduction 2) Pain unpleasantness C = 5 mm, T = 13 mm reduction |
Maximum mouth opening, muscle palpation tenderness |
Maximum mouth opening with/without pain: The increase from baseline to 8 weeks (mm): C = 5/10, T = 3/0
Muscle palpation tenderness (12 points) Baseline: C=132/156 and T=122/156 8 weeks: C=112/132, T=117/156 |
|
Patel et al. 35 |
10/9 (one dropped out later) |
T = BTX, C = saline |
TMD |
Pain scale (1–10) |
Baseline : C = 5.43, T = 5.4 1 month: C = 3.72, T = .9 2 months: C = 1.9, T = 1.3 3 months: C = 3.9, T = 1 |
Incidence of adverse events, Masticatory Muscle Tenderness (composite pain scale (1 = no pain on palpation, 4 = maximal pain on palpation, scale range, 6-24) |
Composite Masticatory Muscle Tenderness Scores
Baseline: C = 15.9, T = 14.9 1 month: C =13.8, T = 8.6 2 months: C = 9.4, T = 8.3 3 months: C = 9.7, T = 9 4 months: C = 12.7, T = 12.5
No adverse effects |
Table 2. Summarized data of the included studies
Results relating to primary outcome measures
In all the five-high evidence RCT, which were included, the pain was assessed as the main factor. The pain assessment was done using visual analog scores (VAS) or a pain scale in the four studies.32-35 In contrast, the research conducted by Kurtoglu et al. used a biobehavioral questionnaire to evaluate their pain associated with TMD. In all the studies, patients received a saline injection in the control group 31, 32, 34, 35 and the test group received a Botox injection. Only in one of the study, a comparison was conducted to evaluate the use of low-level laser and botulinum toxin to treat myofascial pain.33 A survey conducted by Ernberg et al. demonstrated that a significant reduction in pain intensity was observed: BTX-A -30 (33%) after one month and by 23 (30%) after three months compared to 11 (40%) and 4 (33%) for saline.
Kurtoglu et al.31 used a biobehavioral questionnaire where the pain was reduced from 56.1 to 43.9 from baseline after 28 days in the test group. In contrast, in the control group, it decreased from 58.9 to 41.4 from baseline. A study performed by Nixdorf et al. showed a mean change of 19 mm reduction in pain intensity for the BTX-A group, whereas, in the placebo group, a 1 mm reduction was seen regarding baseline. In the study conducted by Carli et al. 33, it was observed that in the laser group, a significant reduction of pain occurred after 12 days of irradiation. In contrast, the group treated with BTX showed decreased pain after 30 days. A study performed by Patel. et al. 35 taught at one month, the composite pain score in the control group was 13.8, whereas, in the test group, it reduced to 8.6 as measured from the baseline.
Results relating to secondary outcome measures
The most common secondary outcome measured was a change in mouth opening after BTX therapy. Kurtoglu et al. measured the anterior temporal and masseters muscles' EMG readings when at rest and during maximal clenching. After the results, it was seen that it reduced from 206.3 mV and 296.0 mV to 165.0 mV and 199.0 mV, respectively, in the test group after 14 days, whereas in the control group, it increased from 200.0 mV to 252.5 mV at rest and from 529.3 mV to 498.8 mV at maximal clenching for the same time interval. Carli et al.33 reported a shift in the maximum mouth opening after 30 days, from 38 to 36, with no change in the control group. Similarly, Nixdorf et al.34 also showed a reduction in the baseline's mouth opening. Ernberg et al.32 reported an improvement in mouth opening as it changed from an initial 42.7 to 44.3 after three months.
Discussion
The focus of our study was to evaluate and assess the available scientific data related to the benefits of BTX in the treatment of TMD. Even though there has been a rising trend of using BTX for managing myofascial pain, still the studies varied in various parameters, e.g., methodology, study design, blinding, intervention. We aimed to collect the data from strong evidence RCT studies where the minimal or moderate risk of bias was present. Therefore, we ensured that from the Cochrane Collaboration's tool 29 the parameters are adequately assessed, and validated outcomes are achieved.
In all the five studies included, the TMD was diagnosed based on specific criteria mentioned in them. The most frequently used tests for the diagnosis of myofascial pain is RDC/TMD. However, using these criteria also has its limitation as it is mainly based on two critical factors, such as myofascial pain and pain accompanied by limited mouth opening. However, there can be a lot of interrelating factors that can lead to TMD and myofascial pain. In the studies, there was heterogeneity in evaluating TMD's diagnosis and other factors related to it, such as occlusal factors, psychological status of the patient, muscle hyperactivity, and other day-to-day functional and social activities.36
Two studies presented a low risk of bias 31, 32, and the rest three showed a moderate risk of bias. The blinding of participants and personnel and outcome assessment parameters was adequately done in the study conducted by Kurtoglu et al. 31 and Ernberg et al. 32 All the five included studies performed the randomization allocation concealment; therefore, a low risk of bias was observed. The majority of studies reported unclear outcome data, which led to a high risk of bias linking to this parameter. By considering the overall risk of bias, the included studies had a satisfactory assessment. The parameters that were not assessed homogeneously in all the reviews should be regarded cautiously in concluding an outcome. There is a need to conduct further investigation to improve the validity of the attained results.
As the primary outcome assessed in all the studies was pain, the findings should be studied attentively. All studies showed that pain was reduced in the groups treated with BTX as compared to the control. However, a study by Kurtoglu et al. 31 and Ernberg et al.32 showed that pain reduction was not significant as not many benefits were observed. At the same time, Nixdorf et al. 34 and Carli concluded that the use of BTX showed minimal benefits compared to the control group. 33 The study conducted by Patel et al. 35 showed that after one month, the pain reduction was more in the group using BTX as compared to the placebo group. However, with time the pain level approached a similar level in both the groups.
The long-term effects of using BTX for pain reduction highlight that it does not significantly improve the symptoms when followed for an extended period. This is due to the various limiting factors that contributed to this outcome. Firstly, in almost all the studies, the sample size was relatively small; the number of study participants was 8-21 in the included studies. The pain scores can be better detected in studies with larger sample sizes, as a relevant significant drop in pain scores might be seen. Additionally, the sample size was not calculated in most of the studies. It is essential to calculate the sample size after explicit sample calculation and power analysis to reduce any probability of random error. 37
The studies were assessed for a short period. Kurtoglu et al., Ernberg et al., Carli et al. 33, Nixdorf et al.34, and Patel et al.35 assessed the pain score for 28 days, three months, 30 days, eight weeks, and four months respectively. The results obtained for such a short study period cannot be applied to larger generalized groups. As follow-up periods were not long enough in the included studies, it does not give an accurate picture of what impact BTX use will have in the medium and long term. If extended for a significant time, the study period will accurately demonstrate the findings, which will give a clear idea about the outcome of pain reduction.
In four studies, VAS was used to evaluate pain.32-35 The VAS is a subjective pain that varies from patient to patient. It shows a 'single-point measurement". Pain is dependent on many other factors; therefore, using VAS is not the most accurate method to assess the degree of pain.38 In all the studies, the patients' pain perception was attained at a particular time, not a reliable approach. A survey conducted by Conti et al. reported that a numeric scale is a better way, which shows greater validity in demonstrating pain value by using a digital level.39 Hence the degree of pain observed before and after the BTX treatment is better understood if a reliable pain scale is used.
In three studies, the BTX was injected in both masseters and temporalis muscle groups.31, 33, 34 In the study performed by Ernberg et al., the injection site included only both masseters muscle groups.32 In contrast, the masticatory muscles - masseter muscles, temporalis muscles, and external pterygoid muscles were injected with the BTX in a study conducted by Patel et al. 35 It is to be noted that pain reduction will be more if more than one masticatory muscle was injected as the effect of the therapeutic agent will be more.
The study conducted by Ernberg et al. 32 assessed physical and emotional function and global improvement as its secondary outcome. It is valuable to find the above factors as pain, directly and indirectly, affects our daily activities, emotional state, and overall feelings. Mouth opening was measured in most of the studies. Mouth opening was reduced as compared to the baseline.
The adverse effect after the treatment with BTX was also reported in the included study. The study by Ernberg et al. 32 mentioned that after BTX injection, they experienced headaches, tiredness or fatigue, jaw pain, and dry mouth. However, these side effects subsided after a 1-month follow-up. Patel et al. 35 said that pain medication usage was more in the placebo group initially compared to the treatment group. These symptoms were reduced with time, and normal functioning was attained among the treated patients. However, the adverse effects such as difficulty in smiling, increasing pain, and tenderness with BTX injections should be noted for a more extended period to achieve an unbiased assessment.
The five studies included in the systematic review showed a lack of apparent outcomes on the effectiveness of the BTX for the treatment of TMD. Therefore, further research is needed to obtain relevant results.
Conclusion
Although the studies included were high evidence RCT with low risk of bias in most of the parameters assessed, the absence of adequate clarity in a few characteristics impacted the overall outcome. To achieve a clear understanding of the benefits of BTX in TMD, it required that RCT with a large sample size, homogeneous study design, longer follow-up period, and based on standardized diagnostic methods are performed.
The results from the included studies in the review support the benefits of BTX in reducing the symptoms associated with TMD, but further research is required to show the effectiveness of BTX. TMD is a multifactorial disorder that impacts an individual's physical, social, emotional, and mental well-being. A targeted approach should be involved after clearly understanding its underlying etiology and then providing the treatment accordingly. Despite their limitations, the studies show that BTX can play a significant role in reducing the pain observed in TMD, which can be justified if more RCT is conducted with larger study sizes.
References
Corresponding Author
Mohammad Abdul Baseer
Department of Preventive Dentistry, College of Dentistry, Riyadh Elm University, Riyadh, Saudi Arabia.
Email: