The Effect of EGF on the Ultrastructure of Submandibular Salivary Glands of Albino Rats Receiving Doxorubicin
Mansy M.1, Soliman Malak2, Mubarak Rabab T.3, Shamel M.4, Kamal Samia M.4
1 Lecturer of Oral Biology, College of Dentistry, Jazan University, KSA.
2 Professor of Oral Biology, Faculty of Dentistry, Cairo University, Egypt.
3Lecturer of Oral Biology, Faculty of Dentistry, BUE, Egypt.
4 Professor of Oral Biology, Faculty of Dentistry, Cairo University, Egypt.
|
|
Introduction
Epidermal growth factor was first purified from the submandibular salivary gland (S.G.) containing low-molecular-weight polypeptide 1 and usually present in many human tissues including submandibular and parotid glands. EGF is a mitogenic polypeptide hormone that in vivo motivates the growth of ectodermal and endodermal cell and in vitro growth of epithelial and fibroblast cells. EGF is a mitogenic polypeptide found in many mammalian species. It had been investigated for its power to accelerate the healing process in several tissues. EGF had shown the ability to activate keratinocyte division in vitro and epidermal renewal in vivo2-4.
The salivary glands, Brunner’s glands of the duodenum and the kidneys are considered as the major physiological source of the EGF production. EGF was a potent motivator of growth for various cell types in vitro and in vivo 5.
The EGF, which is created by platelets, macrophages, and monocytes, interrelates with the epidermal growth factor receptor (EGFR) on epidermal cells and fibroblasts. During wound healing, EGF not only activates epithelial cell growth through the wound but it can also affect fibroblasts and smooth muscle cells. The improvement of recombinant growth factors has upgraded the potential for acute and chronic wound healing. Growth factors give numerous patterns of cell motivation during wound healing. 6-8.
Epidermal growth factor has a powerful curative effect on the gastrointestinal tract. It was also acting as a cytoprotective agent that could stabilize cells against injurious agents such as indomethacin. Also, EGF could avoid hepatic injury caused by carbon tetrachloride and multi organs damage prompted by the thioacetamide 5, 9, 10.
An experiment was conducted on the acini of the parotid salivary glands of rats where apoptosis was induced in the acinar cells with and without the addition of EGF and Insulin Growth Factor (IGF). DNA extraction, immunoblotting, and immunoprecipitation tests revealed that cells treated with EGF and IGF showed suppression in apoptotic cells in comparison to cells without any growth factors. These data indicated that EGF and IGF could be essential in S.G. homeostasis. Furthermore, EGF and IGF could have the power to decrease the unintended apoptosis in salivary glands 11.
Doxorubicin (DXR) is a highly effective chemotherapeutic agent that is widely used in humans and animals. DXR was first purified from Streptomyces species. Idarubicin and epirubicin are synthetically derived as the second generation of DXR. DXR is also isolated from soil actinomycetes (Streptococcus paucities) and was implicated in the treatment of solid tumors including breast, bile ducts, endometrial tissue, esophagus and liver, osteosarcomas, soft-tissue sarcomas and non-Hodgkin's lymphoma 12, 13.
Doxorubicin is a highly effective chemotherapeutic treatment for different cancers 14, 15 such as leukemia, Hodgkin's lymphoma and cancers of the bladder, breast, stomach, lung, ovaries, and others 16 and it has been demonstrated to generate toxicity in the reproductive system 17. It is highly hydrophilic, has a short half-life and its use is usually associated with severe side effects at high doses. Nausea, vomiting, and heart arrhythmias are the most common acute side effects of DXR. Cardiotoxicity is a common side effect of DXR drug caused by nonspecific delivery to the heart 18. Neutropenia (a decrease in white blood cells), as well as complete alopecia (hair loss), were also registered. A milder side effect is discoloration of the urine, which turns bright red after 48 hrs19.
The most serious complication of DXR treatment is its effect on heart cells. DXR may cause dilated cardiomyopathy, congestive heart failure and even sudden cardiac arrest 20, 21. Some studies explained its potential cardiotoxic mechanisms by disruption of energy metabolism, apoptosis, increased reactive oxygen species (ROS) and mitochondrial injury22-26.
ROS produced by the interaction of DXR with iron, destroy the heart cells, causing myofibrillar damage and cytoplasmic vacuolization. Moreover, some cases could develop palmar-plantar skin eruptions that show skin eruptions on the palms of the hand or soles of the feet, swelling, pain, and erythema27.
DXR is widely used to treat different types of human neoplastic disease. Regardless of its high effectiveness; the clinical use of doxorubicin was significantly restricted because of its unwanted side effects. Subsequently, EGF exerts a strong growth and healing effects, it is thought to play a part in the development and renewal of salivary glands and oral mucosa.
The present study was designed to investigate the effect of EGF injection on the cytotoxic effect of DXR induced in submandibular S.G. of rats regarding its ultrastructure.
Materials & Methods
In the present study, we used thirty male adult albino rats, two months old, pathogen-free of an average weight of 200 gm. The rats were obtained from the animal experimental unit, Faculty of Medicine, Cairo University. They have housed five rats per cage, fed the normal laboratory diet and supplied drinking water ad libitum throughout the whole experimental period. The animals were housed in a controlled environment (temperature 25o ± 2 0C, humidity 70 -80 % and 12hr dark/light cycle). The experiment was conducted in the animal house according to the recommendations of the ethics committee on animal experimentation, Faculty of Medicine, Cairo University. The rats were equally divided into three groups (twenty rats each), these groups were categorized as follows:
Control Group: The rats were kept on the normal control diet and did not receive DXR or EGF.
Doxorubicin Group (DXR): The rats received 20 mg/kg body weight Doxorubicin (DXR) as a single intraperitoneal injection 28.
Epidermal growth factor Group (DXR + EGF): The rats received the same intraperitoneal dose of DXR (20 mg/kg body weight) and on the next day they were injected intra-peritoneally by 10µg/kg body weight of EGFprovided by Sigma-Aldrich Inc. 29 for 7 consecutive days.
One day after the last injection, the rats of all groups were euthanized by an intracardiac overdose of sodium thiopental and the submandibular salivary glands were dissected out.
After the glands of the right side were excised, those of the right side were fixed immediately in 10% neutral buffered formalin. Then, the specimens were washed properly under running water, dehydrated through ascending concentrations of alcohol and transferred to xylene to clear the specimens from alcohol. Then, the glands were embedded in paraffin wax and mounted in the center of the paraffin wax blocks. Sections from paraffin-embedded tissue blocks were cut 5 microns thick and mounted on glass slides for histological examination using H&E stain for investigation of the structural features of the salivary gland tissue.
Electron Microscopic (E/M) Examination:
The glands of the left side were prepared and used for the Ultrastructural examination at Cairo University –Research Park CURP 30.
Results:
Light and Transmission electron microscopic examination of the rat submandibular salivary glands of the control group revealed the structural and ultrastructural features of the glands. The lining cells of secretory acini appeared pyramidal in shape with a basally situated rounded nucleus (Figs. 1a). Binucleated cells were not uncommon and the nuclei harbored prominently visible nucleoli. A well-developed system of the rough endoplasmic reticulum (RER) constituted a network around the nuclei. The acinar cell appeared loaded apically by a mass of electron-lucent secretory granules of variable size. The intercellular spaces were occupied by the membranous folds of lateral interdigitation (Figs. 2a).
The intercalated ducts were identified among the secretory units by their cuboidal cell lining containing centrally placed nuclei. Myoepithelial cell grasping the duct appeared with an elongated nucleus. The striated ducts were characterized by the deep infoldings of the basal plasma membrane and the radially oriented numerous longitudinal mitochondria distinguishable by their transverse cristae. They were packed between the membranous invaginations. The central rounded nuclei appeared with granular evenly dispersed chromatin (Figs. 3a).
Histological sections of submandibular salivary glands of rats injected with DXR showed several pathological changes. The secretory acini appeared with massive cytoplasmic vacuolization giving a sieve or cribriform like pattern to the glandular tissue. Also, deformation in the acini and loss of normal cellular orientation were frequently encountered. Moreover, some acini displayed complete degeneration of their secretory cells. A clear space separated the parenchymal elements of the gland so that the acini and ductal profiles were no more grouped in close contact which might be an index of interstitial edema (Fig. 1b).
Electron microscopic examination of the rat submandibular salivary glands revealed alterations in the ultrastructure of the secretory terminal portions and duct system when compared to the control group.
The nuclei of the secretory acinar cells displayed a slight increase in electron density, as represented by chromatin condensation and margination. Shrunken apoptotic nuclei exhibiting nuclear indentation and lobulation were also encountered in the secretory cells on top of chromatin margination and clumping. The mitochondria appeared swollen with disintegrated cristae and indenting the nucleus (Fig. 2b).
The RER was seen grossly dilated, evidence of degranulation, and retained secretion in the cisternae of RER were associated with the destructed mitochondria. Dilated RER cisternae gave a vesiculated appearance to the cytoplasm. Moreover, the secretory granules seemed to be less crowded probably because of the cytoplasmic vacuolizations occupying extensive areas at several sites (Fig. 2b).
The striated duct cells in this group showed a marked reduction in the height and density of the radially arranged mitochondria. Furthermore, the infoldings of the basal plasma membrane seemed to be lost or ill distinguished. Moreover, areas of cytoplasmic vacuolizations together with a reduction in the amount of the apical secretory granules were noticed. Regarding the nuclei, the beginning of nuclear chromatin clumping as an early sign of cell injury was also identified (Fig. 3b).
As compared to the ultrastructural findings of DXR group, the submandibular salivary glands of rat of the immediately treated group with EGF revealed great improvement in the architectural features of the glands (Fig. 1c).
The array of RER thrown in parallel sacs encircled the nucleus without any evidence of cisternal dilatation. The oval bodies of mitochondria almost normal size were distributed all over the cytoplasm and the semicircular stacked flat sacs of the Golgi apparatus were interposed between the array of RER and the secretory granules. The nucleus displayed normal regular arrangement of dispersed chromatin together with a central round nucleolus with no evidence of indentation, lobulation or chromatin margination and clumping (Fig. 2c). The extensive vacuolization was no more detected apart from few microvesicles appearing in the cytoplasm of some specimens.
In the striated ducts, the basal membrane infoldings appeared partially reestablished to separate radially oriented mitochondria that did not attain the size and number previously seen in the control but seemed more abundant than those of DXR group. Nevertheless, an increased amount of apical secretory granules were noticed and appeared with a greater diameter. However, cytoplasmic vacuoles were still being observed (Fig. 3c).
Discussion
Doxorubicin (DXR)is a highly effective chemotherapeutic agent, interfering with the growth of cancer cells by blocking a topoisomerase-2 enzyme needed for the division of these cells. The chemical structure of DXR causes free radicals production and induction of oxidative stress that associates with tissue damage31. DXR also causes a discrepancy between free oxygen radicals and antioxidants. This irregular oxidant-antioxidant system leads to tissue damage that is detected by lipid peroxidation and protein oxidation within the tissue 32.
The results of the present study indicated that the systemic injection of DXR in rats resulted in structural and ultrastructural alterations within the submandibular salivary gland tissue.
The mitochondria were extensively affected; they appeared vacuolated, swollen and losing their cristae. Moreover, the mitochondria at the basal region of striated ducts were markedly reduced in number. It has been documented that in the early stages of cell degeneration, the mitochondria were the first cell organelles that become affected leading to disturbance in the energy production, affecting the activity of the cell and its ability to perform its function properly 33.
The mitochondria might be the leading cause of the intracytoplasmic vacuolizations observed frequently in acinar and ductal cells. Since mitochondria are very weak to harmful agents and if they are damaged, the cellular metabolism fails and sodium ions enter the cell. This osmotic effect induced breakdown of large macromolecules within the damaged cell and accounted for the appearance of cytoplasmic vacuoles. The intracytoplasmic vacuolations had been explained by intracytoplasmic degeneration of other cell organelles, like the Golgi apparatus, that if acquiring fatty nature they appeared as empty spaces 34.
DXR directly diffuses into the cell cytoplasm and binds to the cytoplasmic proteasome subunit (protein complexes that degrade unneeded or damaged proteins by proteolysis). Once the DXR-proteasome complex has entered the nucleus via nuclear pores, it then inhibits topoisomerase II (the key enzyme that maintains DNA tension). Also, DXR intercalates to the DNA strand, preferably at the cytosine-guanine nucleotide pair. These intranuclear processes are considered to be the main mechanisms by which DXR induce cytotoxicity 35.
The submandibular salivary glands of rats injected with DXR and followed by EGF showed great improvement in the gland architecture. The resolution of vacuolar degeneration was noted apart from minute areas of microvesicles detected in the parenchymal tissue. The ultrastructural findings of this group were consistent with the histological examination. Acinar and ductal cells appeared more similar to those of the control group.
Exogenous EGF administration in a neonatal rat necrotizing enterocolitis (NEC) model decreased the expression of IL-18 (a pro-inflammatory mediator) and increased release of IL-10 (an anti-inflammatory cytokine) in the ileum 36. Moreover, EGF was found of clinical value in the prevention and treatment of NEC. It helped to increase healing after inflammation, to decrease bacterial translocation and restore function 37. The anti-inflammatory response of EGF was attributed to its influence on cell adhesion molecules expression and functions 38, 39.
The healing potential of EGF was documented in several studies. It was prevalent in epithelial cell proliferation, migration, re-epithelialization and regeneration of gastric glands during gastric ulcer healing 40, the corneal epithelium 41 and renal epithelium regeneration 42.
These results might be explained by the fact that the EGF binds to its receptor EGFR which leads to autophosphorylation of receptor tyrosine kinase and subsequent activation of signal transduction pathways that are involved in regulating cellular proliferation, differentiation, and survival. EGF enables epidermal cell regeneration and has an essential role in the process of dermal wound healing through the motivation of proliferation and migration of keratinocytes 43.
EGF is a potent cytoprotective and reparative growth factor that is normally formed by distal tubular cells with marked expression during maturation. Furthermore, the protective effect of EGF may be attributed to its mitogenic action which could stimulate the regeneration of the tubular cells 44, 45.
We concluded that EGF has got a cytoprotective and reparative effect rather than a proliferative response. It prevented the cytotoxic effect induced by DXR in the glands at both the structural and ultrastructural levels when immediately supplied following chemotherapeutic injection.
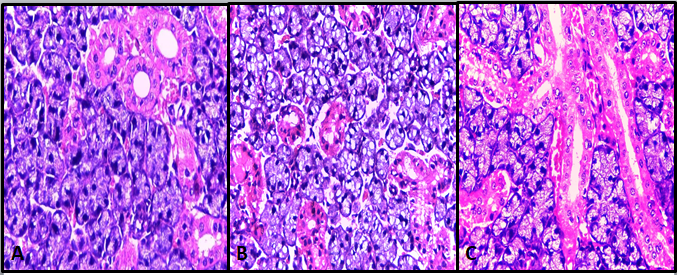
Fig. 1: (A) Photomicrograph of the submandibular salivary gland of rat of control group showing the regular architecture of the acini and ducts (H&E, Orig. mag. X400). (B) Photomicrograph of Submandibular salivary gland of rat of DXR group showing the degenerated glandular tissue with a sieve or cribriform like pattern (H&E Orig. mag. X 400). (C) Photomicrograph of the submandibular salivary gland of rat of Group III showing well-aligned acini and ducts with a rich vascular network in close contact with the excretory ducts, marginated nuclei of the lining cells and few atrophied acini (H&E Orig. mag. X400).
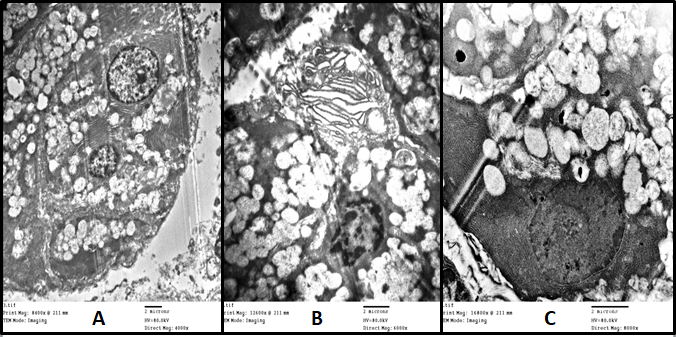
Fig. 2: (A) Electron photomicrograph of the submandibular salivary gland of rat of control group showing a binucleated acinar cell with well-arranged RER and apical secretory granules (Uranyl acetate & lead citrate X4000). (B) Electron photomicrograph of the submandibular salivary gland of rat of DXR group showing a secretory cell with cytoplasmic vacuolization indented electron-dense nucleus with marginated chromatin, dilated cisternae of RER and cytoplasmic vacuoles (Uranyl acetate & lead citrate X6000). (C)Electron photomicrograph of the submandibular salivary gland of rat of EGF group showing the secretory cell with a regular array of RER, oval bodies of mitochondria, the semicircular stacked flat sacs of Golgi apparatus, the regular arrangement of dispersed nuclear chromatin and a central round nucleolus(Uranyl acetate & lead citrate X8000).
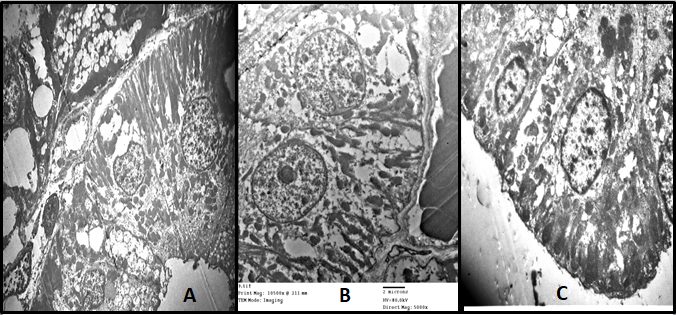
Fig. 3: (A) Electron photomicrograph of the submandibular salivary gland of rat of control group showing the striated duct lining columnar cell with a centrally placed rounded nucleus, numerous longitudinal mitochondria, apical electron-dense secretory granules and wide lumen(Uranyl acetate & lead citrate X2500). (B)Electron photomicrograph of the submandibular salivary gland of rat of DXR group showing striated duct cell with nuclear chromatin clumping, the mitochondria reduced in number and size, cytoplasmic vacuolations and loss of basal membrane infoldings at several sites (Uranyl acetate & lead citrate X5000). (C)Electron photomicrograph of the submandibular salivary gland of rat of group III showing partially reestablished basal infoldings of striated duct separating more plentiful radially oriented mitochondria and increased amount and size of the apical secretory granules (Uranyl acetate & lead citrate X6000).
References
Corresponding Author
Mohamed Hamdy Mansy
Lecturer of Oral Biology, College of Dentistry, Jazan University, KSA.
E-mail:mmansy @ jazanu.edu.sa