|
Original Study |
Comprehensive Review of Oral Granular Cell Lesions
Bhavna Chulliparampil Mohan MDS1*, Punnya V Angadi MDS, PhD2
1 General Dentist and Oral Pathologist, AL Afdal Medical Center, AL Mahatah, Sharjah, United Arab Emirates.
2 Professor and Head of Department, Department of Oral Pathology and Microbiology, KLE VK Institute of Dental Sciences, KLE Academy of Higher Education and Research, Belgaum, Karnataka, India.
|
|
Introduction
Classification and understanding of lesions using various cell shapes and cytoplasmic changes is routine often used by pathologists to narrow down to a diagnosis. Some of the common cytoplasmic changes noted are clear, granular, fibrillar, rhabdoid, oncocytoid, and hyaline cell appearances.1Among these, granular appearances are studied in great detail, yet the true nature of this granular appearance, the cause, its significance, and prognosis remains elusive.1-6
Reasons for granularity in oral lesions
Cellular cytoplasm usually has an eosinophilic appearance in hematoxylin and eosin (H&E)-stained sections due to the presence of cationic (basic) structures in the cytoplasm, which take up acidic eosin. The majority of cytoplasmic organelles like mitochondria, lysosomes, smooth endoplasmic reticulum, cytofilaments, and some neuroendocrine and exocrine granules take up acidic eosin. Abnormal increase of anyone or a combination of these organelles in the cells is the prime cause of the granularity seen in different lesions.7 This increase may be due to degenerative changes or a result of cellular adaptation to stress resulting in increased organelles. The former is often the case with lysosomes6 and the latter is noted with mitochondrial proliferation in stressful conditions.7 In various pathologies the increased cell organelle is different. In most pathologies, granularity is mainly due to the increase in lysosomes or mitochondria. However, in liver pathology, the classical ground glass hepatocyte appearance is due to the increased endoplasmic reticulum or free ribosomes in the cytoplasm.8 The alternative increase in neuroendocrine granules is seen in neuroendocrine tumors.9 Other individual cells also show granularity like the melanocytes with melanin granules10, serous acinar cells with zymogen granules11,12, haemosiderin laden cells, and granulocytes like eosinophils and basophils. Some of the reasons of granularity are described below in greater depth.
1. Lysosomal aggregation
In the oral cavity, secondary granular cell changes are seen in a heterogeneous group of lesions like soft tissue tumors, odontogenic tumors, reactive lesions, and also in immune-mediated diseases like lichen planus. In most lesions, granular cell phenotype seems nonspecific for a given tumor type, but rather represents a peculiar cellular change characterized by a marked increase of the lysosomal component.13-18
This lysosomal increase may be due to intrinsic metabolic alterations and dysfunction of tumor cells3,17, autodigestion of cell constituents leading to accumulation of autophagolysosomes14, adaptive changes in response to stress16, formation of intracytoplasmic crystalloids due to cellular degeneration18, senescent changes17, apoptotic cell death proceeded by phagocytosis by adjacent neoplastic cells2, 19, etc. The other theory suggested is that lysosomes in the cytoplasm are an active component and they are involved with the active remodeling of the cytoplasm rather than a degenerative change.15
In granular cell tumors, the myelin figures are explained by the assumption that Schwann cells acquire lysosomes during phagocytosis of myelin, a phenomenon known to occur especially in peripheral nerves showing Wallerian degeneration as well as in traumatic neuromas.13,14 Increased lysosomes has been proved to be the cause for the majority of oral granular cell lesions (Table 1). In the odontogenic epithelium, an increase in autophagic lysosomes has been observed in ameloblasts during normal amelogenesis.20, 21 Certain developing tooth sections have shown an accumulation of cells associated with enamel organ suggestive of a lysosomal insufficiency or due to an overproduction of unused materials in the odontogenic epithelium.2,20,21 Few authors have hypothesized that granular cell change in ameloblastoma may be due to lysosomal aggregation in tumor ameloblasts to digest unwanted components.2,17
The significance of lysosomes still remains controversial. The mechanism of granular change and its prognostic significance is unclear.22-24 Usually, they do not affect the biologic behavior of most soft tissue tumors but in certain tumors, like granular cell ameloblastoma the granular appearance has been associated with more locally aggressive behavior23,24 and a recurrence rate of about 33% was noted.
2. Histiocytes/macrophage
2a.) Histiocytes are cells of either the macrophage or Langerhans cell lineages. The histiocytic disorders are characterized by the proliferation of cells of these lineages.25 Their cytoplasm is eosinophilic and contains variable amounts of lysosomes. The histiocytes include macrophages and dendritic cells.26 Macrophages are highly variable in size and morphology, their cytoplasm contains numerous acid phosphatase laden lysosomes in relation to their specialized phagocytic function 27,28. In addition to studies by Miller et al.,16 various studies have indicated that granular cells also contain histiocytic markers like α-1-antitrypsin, antichymotrypsin, lysozyme, CD-68, and HLA-DR, which are all characteristic of histiocytes.3 Reactive granular cell lesions containing histiocytes is a separate lesion and is usually seen in sites of trauma.29
2b.) Ceroid laden macrophages were identified as the cause for granularity in a series of oral lichen planus with unusual histology containing finely granular cells in the lamina propria.30, 31 Based on cytological and cytochemical studies, it was found that these granular cells are macrophages of low proliferative activity laden with early ceroid.31, 32 Ceroid and lipofuscin are two pigments seen in aging and degenerating tissues. It was proposed that ceroids are residual lysosomal bodies containing cross-linked aggregates of damaged cellular components.33 In this situation, the damaged basal keratinocytes provide debris for the formation of this ceroid material resulting in the granular cells seen in oral lichen planus. It is suggested that oral ceroid granuloma would be an appropriate term for collections of such reactive macrophages31 seen in the oral tissues.
2c.) Xanthoma- Xanthomas are lesions characterized by the accumulations of lipid-laden macrophages. This is most commonly seen in verruciform xanthoma of the oral cavity.34, 35
3. Mitochondria
The cytoplasmic eosinophilia caused by increased mitochondria is defined as oncocytic change. Hamperl was the first to recognize this phenomenon and he initially coined the term oncocytes to identify such granular cells in salivary glands, thyroid, parathyroid, gall bladder, adrenal gland, and pituitary gland.36 These cells with increased altered and engorged mitochondria are called as oncocytes and this oncocytic change is of significance as it is most often seen in the salivary glands as a focal or predominant feature.36-56 Oncocytic-like lesions constitute another group of lesions in which the eosinophilic granularity is associated with the increase in organelles other than mitochondria like ribosomes, lysosomes, etc.
In the salivary gland, the oncocytes may represent a reactive, benign, or malignant neoplasm. Reactive proliferations are often seen in salivary glands and are referred to as oncocytosis. In neoplasms like oncocytoma, Warthin’s tumor, and oncocytic carcinoma the histogenesis of the tumor is suggested to be from the striated duct. The striated duct is known to have three times more mitochondria than the other ducts.57 This increase in mitochondria may be the probable cause for an oncocytic appearance in these tumor cells.
4. Zymogen granules
These are most often seen in acinic cell carcinoma 11,12, which is a low-grade salivary gland malignancy. On the basis of ultrastructural observations, acinic cell carcinoma is thought to be derived from the serous acinar proliferation.11,12
Theories for granular cell appearance in tumors
In the human body, granular cell changes have been previously reported in a variety of neoplasms like neurofibromas, dermatofibromas, leiomyomas, schwannomas, leiomyosarcomas, angiosarcomas, fibroxanthomas, oligodendromas, astrocytomas, etc.58 Many benign, malignant, and reactive lesions that occur in the oral cavity also contain granular cells as a characteristic component of their pathology.59 The predominant oral granular cell lesions that have been identified are listed in Table 1 based on the main causes of granularity in these lesions. 60-100 The major causes identified are lysosomal aggregations, oncocytic lesions, histiocytic lesions, and other rarer miscellaneous lesions.
The causes of granularity have been investigated and previously it was postulated that tumors containing granular cells were of two main types.58 One in which the main population is of granular cells, as in a neoplasm composed predominantly of granular cells and the second as a localized change in a neoplasm of another recognizable cell type. In the present review, the granular cell changes in oral lesions are categorized into four main possibilities.
Neoplastic theory
Granular cell myoblastoma or granular cell tumor represents a tumor whose histological hallmark is the presence of a predominant population of granular cells. This tumor has an uncertain histogenesis.3-5, 101-105 Earlier this tumor was assumed to be of myoblastic origin however recent molecular and ultrastructurally studies have ascertained that granular cells in this neoplasm are most commonly considered to be of Schwann cell origin.101-104 Still newer evidence points to a neuroendocrine origin for these granular cell tumors.4,105
Other tumors that can be classified under neoplastic theory include neuroendocrine tumors like neuroblastoma, phaeochromocytoma, Merkel cell tumor, parathyroid adenoma, and so on. The neoplastic proliferation of cells with the neuroendocrine granules imparts the granular appearance.9 Langerhans cell histiocytosis represents the neoplastic proliferation of malignant histiocytes leading to granular appearance.25, 106 Melanoma is another example where the proliferating melanocytes with melanin granules are the cause for granularity.10
Among salivary glands, the oncocytoma, oncocytic carcinoma, and acinic cell carcinoma usually have a classic granular appearance.42, 43, 48, 58-60 In oncocytomas, it may be suggested that the neoplastic cells of the striated duct with increased mitochondria contribute to the granularity57 whereas, in acinic cell carcinoma, granularity is pointed to the abnormally proliferating serous acini composed of zymogen granules.58-60
Secondary event theory
The origin and reason for granularity in granular cell tumors were studied extensively to understand the true nature of the granular cells, their prognostic significance, and to know if the granular appearance in these tumors is similar to the focal granular cell changes seen in other neoplasms. The granularity is due to presence intracytoplasmic granules, which represent lysosomes, similar to the degenerative phenomenon seen in other granular lesions.10-15 Based on these findings, it was suggested that granular cell tumor or the congenital epulis need not necessarily represent a pure neoplasm composed of granular cell proliferation, rather it represents a cytological phenotype of granular cell transformation in the neoplastic cells due to certain metabolic alterations resulting in lysosomal aggregation.3, 5, 107 This granular appearance, which is seen in a diverse group of lesions is thought to represent a secondary event, which is the most accepted theory to explain granular cell transformation.5, 22 This is also supported by the fact that a wide variety of tumors, most of them having a mesenchymal origin, is accompanied by a granular cell component.7 Most of the granular lesions are usually due to the aggregations of lysosomes as a secondary change.
Other secondary events include increased mitochondrial accumulation as seen in oncocytosis and oncocytic tumors. In the salivary gland, this metaplastic replacement is suggested due to an age-dependent metabolic defect.108 This is due to the depletion of mitochondrial enzymes with age and associated compensatory hyperplasia. In oncocytic tumors like oncocytoma, a few authors have suggested that the merging of multiple foci of oncocytic change or oncocytic secondary change in a primary adenoma may be the cause for these tumors.43, 47-52 By and large numerous salivary gland neoplasms exhibit oncocytic metaplasia as a secondary event.47-52 Oncocytic change as a secondary phenomenon has also been reported in inflammatory fibrous hyperplasias.53, 54
Collision theory
Other less significant hypothesis put forward to explain the granular cell appearances in neoplasms was the concept of a collision tumor. Collision theory refers to the development of two separate and unrelated neoplasms that happened to be in close anatomic proximity of each other, which eventually merged into a single mass.7 Such instances have been reported in a few cases where granular cell tumors were seen in association with esophageal carcinoma81 and another case of tongue squamous cell carcinoma.82 Though in most of the cases, this synchronous occurrence of two different tumors was considered coincidental, a few authors suggest that such an association may not be just a cursory finding.82
Dedifferentiation theory
The dedifferentiation theory suggests that granularity is due to the dedifferentiation of the primary tumor into progenitor cells with more granular appearance.7 This hypothesis was earlier applied to granular cell tumors stating that the origin was from myoblasts, which have a granular appearance.101, 102 However, recent research has shown that this lesion is of Schwann cell origin with a focal lysosomal defect causing the granularity.101-105 This theory could, however, be of significance in explaining the granularity in lesions like rhabdomyoma and rhabdomyosarcomas where during the neoplastic process, the cells dedifferentiate into more progenitor cells called the myoblasts, which may have a granular cell appearance.
Identifying oral granular cell lesions
The presence of a variety of lesions showing granular cell appearance leads to diagnostic confusion in many cases as the histopathological and electron microscopic appearance may be similar. In such cases, the use of immunohistochemical markers is helpful in differentiating between the various tumors having granular cell appearance.
Understanding the cause of granularity provides better clarity on the panel that needs to be used in its identification. Granular cell tumors are rare lesions, which usually occur in middle age and the tongue is most commonly affected. Granular cell tumor shows predominant S-100, vimentin, PGP9.5, neuron-specific enolase (NSE), and lysosomal markers positivity.3-5, 14, 16, 24 These findings predominantly suggest a neural cell origin or a neuroendocrine origin for granular cell tumors.4, 5 The congenital epulis is usually seen in newborn children and immunohistochemical studies have revealed that it has lesser or no S-100 positivity in comparison to granular cell tumors suggesting its origin from nonneural mesenchymal cells.59, 64 Though granular cell ameloblastomas represent abundant lysosomal positivity, in contrast to granular cell tumors, they show cytokeratin positivity suggesting their epithelial origin.2, 15, 17, 18 The central granular cell odontogenic tumor has granularity and contains both epithelial and mesenchymal components in abundance and the granular cells are vimentin-positive, suggestive of a mesenchymal origin.74 The oncocytic lesions can be identified based on the positivity for mitochondrial antigens.36, 42, 43 The PTAH stain is also useful in identifying the mitochondria. The neuroendocrine tumors show intense positivity to neural markers like neuron-specific enolase, PGP9.5, and chromogranin.9 Rhabdomyomas and rhabdomyosarcomas can be identified by their positivity for markers like desmin, actin, and myoglobin.91, 92
Other rarer lesions that require confirmation are alveolar soft part sarcoma where the crystalline granules and crystalloids are positive for MCT 1 and CD 147.87 The amelanotic melanomas are other lesions, which cause confusion histologically due to their granular cytoplasm. The diagnosis for these lesions can be confirmed by their positivity for S-100 and HMB-45.10 Lesions like Langerhans cell histiocytosis are identified by the CD-1A positivity and other histiocytic markers.25 hence, as a conclusion, granular cell lesions represent a diverse group of lesions with different causes that contribute to its granularity. A proper understanding of the nature of these granular cells will help to narrow down to an accurate diagnosis in cases of uncertainty.
References
Corresponding Author
Dr. Bhavna Chulliparampil Mohan MDS
General Dentist and Oral Pathologist
AL Afdal Medical Center, AL Mahatah,
Sharjah, United Arab Emirates.
Email: bhavnachulli @ gmail.com
Classification of Reported Types of Oral Granular Cell Lesions Based on Cause of Granularity
|
Cause of granularity |
Reference |
|
|
|
|
|
6,16,23,24 |
|
60,61 |
|
62-64 |
|
65 |
|
66 |
|
67-69 |
|
69-71 |
|
72-74 |
|
75,76 |
|
77 |
|
|
|
78,79 |
|
80 |
|
81,82 |
|
83 |
|
84 |
|
85-87 |
|
|
|
|
|
25,88 |
|
|
|
34,35 |
|
|
|
30,31 |
|
29 |
|
|
|
|
|
7,42, 43 |
|
43,44,89 |
|
37-40 |
|
41 |
|
|
|
48,89 |
|
|
|
46 |
|
43 |
|
43 |
|
43 |
|
90 |
|
45 |
|
|
|
47 |
|
49 |
|
52 |
|
43 |
|
43 |
|
50,51 |
|
|
|
91,92 |
|
93 |
|
94 |
|
55 |
|
95 |
|
|
|
|
|
96 |
|
97 |
|
98 |
|
|
|
58-60 |
|
10 |
|
99 |
|
100 |
|
GCT: granular cell tumor SCC: squamous cell carcinoma SGL: salivary gland lesions |
|
|
|
|
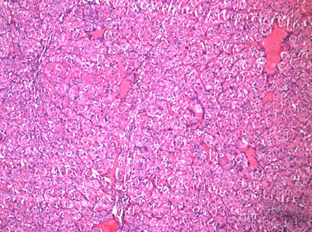
Figure 1: Acinic cell carcinoma 10x
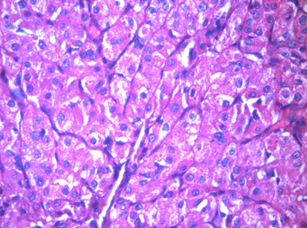
Figure 2: Acinic cell carcinoma 40x
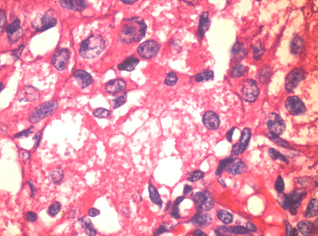
Figure 3: Acinic cell carcinoma 100x