The Role Of PET-CT In The Diagnosis Of Carcinoma Of The Buccal Mucosa With Severe Trismus- A Case Report
Karthik R1*, Mohan N2, Ravikumar PT3, Saramma Mathew Fenn4, Kumar A5, Sabitha Gokulraj6
1 Department of Oral Medicine, Diagnosis and Radiology, Vinayaka missions Sankaracharya Dental College, Vinayaka Missions Research Foundation, Salem, Tamilnadu, India.
2 Head of Department, Department of Oral Medicine, Diagnosis and Radiology, Vinayaka Missions Sankaracharya Dental college, Vinayaka Missions Research Foundation, Salem, Tamilnadu, India.
3 Additional Professor, Department of oral medicine, diagnosis and radiology, Vinayaka missions Sankaracharya Dental College, Vinayaka missions Research Foundation, Salem, Tamilnadu, India.
4 Additional Reader, Department of Oral Medicine, Diagnosis and Radiology, Vinayaka Missions Sankaracharya Dental College, Vinayaka Missions Research Foundation, Salem, Tamilnadu, India.
5 Senior lecturer, Vinayaka missions Sankaracharya Dental College, Vinayaka Missions Research Foundation, Salem, Tamilnadu, India..
6 Additional reader, Vinayaka Missions Sankaracharya Dental College, Vinayaka missions research foundation, Salem, Tamilnadu, India..
|
|
Introduction
Case Report: A 40-year-old male reported to our department of oral medicine, diagnosis, and radiology with a chief complaint of inability to open his mouth and associated difficulty to eat the food. On eliciting the history, the patient had the habit of tobacco chewing in the form of Gutkha for the past 25 years and usually kept it as a quid within the right mandibular buccal vestibule. The patient had progressive difficulty in mouth opening for the past 7 months and at present was unable to open his mouth. Health is very importantessential to us 1. The subject of health is one of the leading priorities of individuals' lives and their satisfaction with the quality of provided health services is an important issue 2. Pharmaceutical services are a critical component of Primary Health care 3. Quality of life is the generaloverall well-being of individuals and communities, outliningdelineating negative and positive aspects of life 4.
Extra-oral examination indicated only a sunken appearance of the face and did not demonstrate any facial asymmetry. (Fig 1) The interincisal distance recorded was only 0.5 mm in diameter. Blanching was seen on the maxillary labial mucosa, a palpable fibrotic band felt during palpation of the right buccal mucosa associated with bleeding.
Considering the pale blanched appearance of oral mucosa and the presence of a palpable fibrotic band on the right buccal mucosa and severely restricted mouth opening, a provisional diagnosis of oral submucous fibrosis associated with trismus was made. Oral submucous fibrosis is a possibly malignant disorder with a chronic insidious onset, described by progressive fibrosis of any part of the oral mucous membrane leading to reduction in mouth opening and progressive inability to open the mouth and sometimes fibrosis of pharynx resulting in dysphagia or fibrosis of eustachian tube leading to conduction deafness or hyperacusis because of involvement of fibrosis of stapedius muscle.
The patient was subjected to PET-CT scan, as he was not capable to subject to conventional radiographic examinations like orthopantomography and unable to tolerate intra-oral periapical radiograph due to severely limited mouth opening. The patient was not diabetic. A Whole-body PET/CT scan from the vertex to mid-thigh region was performed following intravenous injection of 10.3 mCi of 18F FDG (Fluorodeoxyglucose) with a Siemens biograph Horizon PET-CT system without breath-holding instruction (Fig 2). PET scan revealed an area of 0.5 mm thickness increased uptake of FDG (Fluoro-deoxy-Glucose) on the right buccinator muscle and right masseteric space extending into the retromolar trigone region, suggesting the diagnosis of Buccal mucosa carcinoma invading the masseter muscle and extending into the retromolar trigone. There was no erosion of the underlying cortical bone (Fig 3).
Axial section of the PET-CT Scan revealed increased uptake of 18FDG within the vicinity of the right buccinators muscle and deep parts of the masseter muscle region. There was no buccal or lingual cortical bony erosion or any metastatic lymphadenopathy or increased uptake of FDG in the lymph nodes near the Buccinator and masseter muscle (Fig 4). The treatment strategies for the buccal mucosa carcinoma include surgical en-block resection with mandibulectomy followed by surgical reconstruction through latissimus dorsi flap.
The latissimus dorsi free flap is favored as it has a richly vascularized muscle with the largest potential surface area, providing satisfactory bulk and coverage for any defect in the orofacial region 5. The most destructive carcinoma is the Buccal mucosa squamous cell carcinoma 6. Crossed Pectoralis Major Myocutaneous Flap can be utilized safely. It was dependable for the reconstruction of the buccal mucosal defect and in particular patients even for full-thickness cheek defect as folded Bipaddle Pectoralis Major Myocutaneous Flap 7. Surgical techniques for resection of buccal mucosa involve masticator space.
The Weber-Ferguson incision and its alterations were introduced as an anterior methodology to the maxilla, and have the main weakness of unaesthetic facial incision and post-surgical bone defect. Conley's lateral methodology of extending the preauricular incision to the neck with a second submandibular incision was recommended but the approach has weaknesses of facial incision and bony defect along with the sacrifice of inner deep structures. Castro et al suggested a preauricular and transcervical incision approach to malignant tumors of the masticator space, however, it has the potential danger of involvement of facial nerve trunks 8. Dingman and Conley proposed an inferior attitude through the submandibular incision, in which a midline lip splitting and posterior extension to the mastoid process. Direct access to the pterygomaxillary region is probable after horizontal osteotomy of the ascending ramus of the mandible 9. Spiro et al suggested mandibular “swing” approach for the mandible which includes a lip-splitting incision that extends from the mentum-to-mastoid portion, and median mandibulotomy with para lingual extension offers more versatility and good local control for tumors involving the Buccal mucosa 10. John et al suggested that surgical intervention of treatment of Buccal mucosa carcinoma can affect speech and swallowing 11. Giri et al recommended (Teletherapy) External beam radiotherapy by An anterolateral wedge pair technique by telecobalt at a Source surface Distance (SSD ) = 80 cm or a single ipsilateral portal of doses 4,400-5,000 cGy for 4-5 weeks at 2 Gy per fraction from telecaesium unit (Caesa-Gammatron at a source-surface distance (SSD) =40 cm or Brachytherapy by interstitial implantation of preloaded 137 Cs needles) under general anesthesia percutaneously in the Gingivo Buccal sulcus groove with at least 1cm distance between the needles to guarantee satisfactory coverage of the tumor 12. Hopkins Criteria can help in aiding correct assessment of treatment response, especially when scan findings are interpreted as equivocal 13. Hopkins criterion is a 5-point criterion that can be used to assess therapeutic response after radiation with or without chemotherapy in head and neck cancer patients (scores 1-3 were considered negative for residual disease, with a score of 3 considered likely due to post-radiation inflammatory uptake; whereas scores 4-5 were considered positive) 13. Table 1 enumerates the various research studies on Buccal mucosa carcinoma in India.
|
Author |
Year |
Study subjects |
Results |
|
Singh et al 14 |
1966 |
362 Patients with Carcinoma in the Buccal mucosa |
30% treated with radiotherapy and 9% treated with surgery |
|
Von Essen et al 15 |
1968 |
100 Patients |
20-30% tumor regression seen in patients treated with Methotrexate and 5-Fluorouracil chemotherapy |
|
Krishnamurthy et al 16 |
1971 |
927 Patients |
39% of patients had the disease under control after treatment |
|
Nair et al 17 |
1988 |
234 Patients |
Overall survival rate of 42% for stage I, 85% for stage II, 63% for stage III, 41% for stage 1V |
|
Borges et al 18 |
1989 |
71 Patients |
Tumor thickness greater than 5mm associated with nodal metastases has a very poor prognosis and carcinoma of Buccal mucosa is aggressive and has different biological behavior. |
|
Pradhan et al 19 |
1989 |
|
PORT better than surgery alone. Adjuvant therapy is essential for carcinoma of Buccal mucosa |
|
Bahadur et al 20 |
1992 |
252 Patients |
The combined use of pre or post-operative radiotherapy and radical surgery in Buccal mucosa carcinoma; the absolute and determinate survival rates were 55% and 61%, respectively. |
|
Mishra et al 21 |
1996 |
|
Postoperative radiotherapy 68% improved survival rate |
|
Mishra et al 22 |
1999 |
176 Patients |
T2 stage of Buccal mucosa carcinoma is associated with a high failure rate, and needs adjuvant therapy. |
|
Iype et al 23 |
2001 |
264 Patients |
Higher T stage of Buccal mucosa carcinoma was associated with a local failure rate. |
|
Yeole et al 24 |
2003 |
1808 Patients |
30% survival of carcinoma of Buccal mucosa and retromolar trigone is poorer. |
|
Iyer et al 25 |
2004 |
46 Patients |
61% of Buccal mucosa carcinoma who were nonsmokers responded worse. |
|
Badakh et al 26 |
2005 |
94 Patients |
Patients with positive surgical margins responded poorly. The dose of 60 Gy is not enough in postoperative radiotherapy. |
|
Singhania et al 27 |
2015 |
13,500 cases in Indian cancer registry- a retrospective study |
Buccal mucosa carcinoma is attributable to the utilization of tobacco products in India. |
|
Padma et al 28 |
2017 |
125 males and 73 Females |
The most common complaint in Buccal mucosa carcinoma patients is pain followed by bleeding. |
|
Bobdey et al 29 |
2018 |
409 Pathologically proven Buccal mucosa cancer patients and treated surgically in Tata Memorial Hospital |
Buccal mucosa carcinoma is an aggressive malignant tumor. The total 5-year survival rate was 54.1%. |
Table 1. The various research studies on Buccal mucosa carcinoma in India.
The Indian council on medical research suggested that the minimum required post-operative Radiotherapy dose is 60 Gy at 1.8-2 Gy/fr. This may be provided in a phased manner. The initial phase would deliver 44Gy in 22 fractions over four and a half weeks to the primary tumor areas using conventional treatment planning, 3DCRT (3-Dimensional conformal Radiotherapy), or IMRT (Intensity Modulated Radiotherapy) 30. Brachytherapy may be delivered by means of low dose rate brachytherapy (LDR) of 65-70Gy/6-7 days or high dose rate Brachytherapy (HDR) of about 48Gy/12fr 4Gy BD x 6 days. 30 Patients who are not appropriate for brachytherapy may be treated with EBRT (External Beam Radiotherapy).
EBRT is delivered utilizing conventional planning /3DCRT/IMRT to doses of 66-70Gy at 1.8 to 2 Gy per fraction over 7-8 weeks (or a biologically comparable dose) with suitable margins all around the tumor. In conventional radiotherapy, a dose of 44Gy in 22 fractions/over 4.5 weeks, followed by 12-16 Gy is suggested after shielding the spine.
The recommended dose of external beam radiotherapy for buccal mucosa carcinoma was 45-50Gy if interstitial boost {dose of 20-25 Gy (LDR) or equivalent HDR)} is given 30. Cisplatin in doses of 100 mg/m2 every three weeks is recommended every three weeks on days 1, 22, and 43 of radiotherapy 31.
Limitations of PET-CT: PET-CT cannot be performed in patients with poorly uncontrolled diabetes of postprandial blood glucose level more than 220 mg/dl, as such patients give a false positive uptake of 18-Fluorodeoxyglucose when subjected to PET scan. False-positive uptake of FDG also occurs due to inflammation caused by post-surgical resection of carcinoma or post-radiation therapy. Hence, it is advisable to take a follow-up scan only after 8-12 weeks post-surgery or radiotherapy. PET scan cannot appreciate metastatic necrotic lymph nodes caused by squamous cell carcinoma 32.
Clinical significance: Patients with trismus cannot be ignored during a clinical examination. PET Scan is important in the diagnosis of Buccal mucosa carcinoma in patients with inadequate visual examination due to severe trismus, which may hamper adequate clinical and radiological examinations. FDG PET/CT has excellent diagnostic accuracy for detecting locoregional nodal and distant metastases of lymph nodes less than 1cm, which are not appreciable well in CT scans and PET/CT scan can be utilized to measure therapeutic response and provides valuable information about prognosis in patients with oral cavity cancer.
References
Corresponding Author
Karthik R
Reader, Department of Oral Medicine, Diagnosis and Radiology, Vinayaka Missions Sankaracharya Dental College, Vinayaka Missions Research Foundation, Salem, Tamilnadu, India.
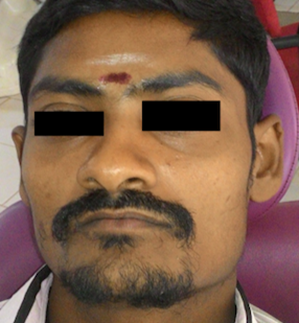
Figure 1. Extraoral profile
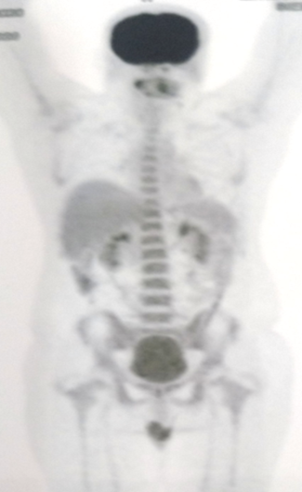
Figure 2. Whole body PET Scan
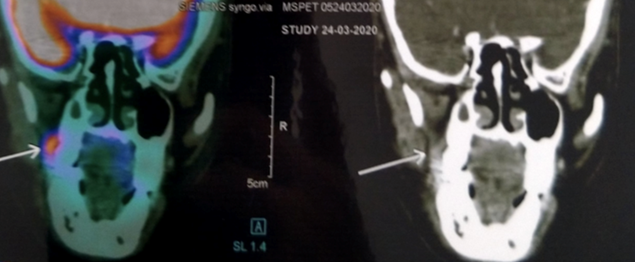
Figure 3. Coronal section of PET-CT Scan image
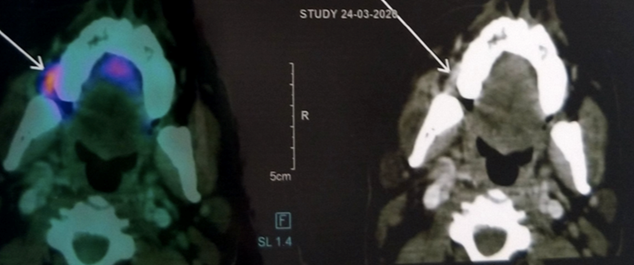
Figure 4. Axial section of PET_CT Image