Comparative Effect of Contamination and Decontamination of Two Adhesive Systems by Blood and Haemostatic Agent on the Bond Strength to Dentin: In Vitro
Aseel Fareed Faisal 1, Shibu Thomas Mathew 2*
1 Postgraduate, Restorative Dentistry, Riyadh Elm University, An Namudhajiyah, Riyadh 12734, Saudi Arabia.
2 Assistant Professor, Riyadh Elm University, An Namudhajiyah, Riyadh 12734, Saudi Arabia.
|
|
Introduction
Dentin bonding is an extreme complex when it is compared to enamel bonding. Consequently, studies concerning the bonding efficacy of saliva-contaminated dentin bonding agents are controversial1. Extensive researches have been carried out on the the effect of saliva contamination on the bond strength of composite to tooth structure. the results of the researches vary in different researches due to the different bonding system. However, the effect of blood contamination on the adhesive properties of self-etch and total eth adhesives has not been fully understood.
Composite resins are technique-sensitive, and achieving good isolation is very important2. With increasing demand and using of aesthetic restorations, contamination control has been a much-debated and important topic since adhesives systems and composite are very vulnerable to contamination3. Isolation of the operative field is a prerequisite for the success of the esthetic treatments. Achieving this is sometimes a common problem especially when rubber dam isolation is not feasible 4.
Several types of contamination can affect the structural and chemical properties of dental restorative materials, such as blood, saliva, crevicular fluid and haemostatic agents5. Recently developed adhesive systems such as total-etch and self-etch system have reduced the number of application steps and thus reduced the risk of contamination in the field of operation. Several studies have suggested that “total-etching single bottle adhesive systems” are less sensitive to contamination with saliva than previous-generation binding agents 5-7.
Regardless of the time of contamination, to recover the original bond strength values, different decontamination procedures have been used on contaminated 7, 8. Some studies show that decontamination procedures can recover bond strength6,8, but others show the contrary 1. Then, it seems possible that decontamination after blood contamination could increase bond strength. Elevated blood pressure, is a critical hazard factor for the cardiac accident which incorporate coronary artery diseases (CAD), stroke, congestive heart failure, cerebro-vascular accident, peripheral arterial insufficiency and end-stage renal disease. 9 A re-etching, water rinsing or adhesive reapplication is some of this techniques 8.
Fewer studies investigated the effect of blood and haemostatic agent contamination on the bond strength of resin by one bottle system of adhesive application to dentin. It, therefore, seems that investigations that elucidate the effect of blood and haemostatic agent contamination on dentin bond strength on recently developed one bottle system adhesion would provide practical knowledge of great use to a dental professional.10, 11
In wake of these concerns, the aim of this study was to investigate the influence of blood and haemostatic agent contamination of dentin during Bonding procedure on shear bond strength and to investigate the effect of different decontamination treatments on bond strength of two new bonding agents Prime & Bond Select and Prime & Bond Etch & Rinse.
Materials and Methods:
130 freshly extracted sound human premolars and molars were cleaned and stored in 0.1% thymol for a week and in distal water until they were used in this study. Carious teeth, fractured teeth and restored teeth were excluded from the study. Fresh capillary blood was collected from a single individual at the same site and time, as the specimens were made. Haemostatic agent (Dharma, 25% Aluminium Chloride) was also used in this study.
The roots of 130 teeth were cut-off with a double-faced diamond disk (KG Sorensen, Barueri, Brazil), under running water, and any tissue remnants and debris were removed. All crown portions were retained, flat dentin on the buccal side was reduced by 2 mm, and the dentin was cut with Isomet machine and ground using Automata machine with wet 600-grit SiC paper (Norton Abrasives, Campinas Brazil) under running water. After cleaning and air drying, the teeth were then mounted in acrylic jigs of standard diameter and filled with plaster, any excess material was removed.
The experimental specimens were randomly divided into 2 groups according to the adhesive used.
Contaminant application:
Blood: was collected from the fingertip of the same individual (needle prick of an alcohol wipe for finger). One drop of blood was applied directly to the surface of each specimen and left undisturbed for 20 seconds (Van Schalkwyk, Botha et al. 2003).
Haemostatic: was applied for 20 seconds, surface rinsed with distilled water and air-dried for 3-5 seconds.
Decontaminant application:
Rinse and dry treatment were performed by spraying water 10 cm from the target tissue for 10 seconds, and then a gentle blast of air until the substrate was completely dry (Sattabanasuk, Shimada et al. 2006). Conversely, as a gentle air spray 10 cm from the target tissue for 20 seconds, the blot dry procedure was performed to produce a dry layer of blood (Faltermeier A, Behr et al. 2007). NaOCl was applied by syringe and remain in contact with the surface for 20 seconds, followed by gentle air spray for 20 seconds.
Group 1 (n=65): Self-etch adhesive Prime & Bond Select (Dentsply Detrey GmbH, 78467 Konstanz, Germany). The adhesive application was applied with a disposable brush (Kerr Corporation, Orange, CA, USA), left untouched for 20 seconds, and light-cured.
Group 2 (n=65): Total-etch Prime & Bond Etch & Rinse (Dentsply Detrey GmbH, 78467 Konstanz, Germany). The dentin surface was first etched with 37% phosphoric acid gel
Total etch (37% phosphoric acid), for 15 seconds, rinsed with distilled water for another 15 seconds and dried with moist cotton pellet. Then, Adhesive application was applied with a disposable brush (Kerr Corporation, Orange, CA, USA), left untouched for 20 seconds, and light-cured.
65 molars and premolars in each adhesive group will be further sub-divided among six experimental groups per adhesive type.
Specimen distribution:
Control Group 1A (n=5): Without contamination. Self-etching adhesive was applied to dentin according to the manufacturers' instructions and light-cured for 10 seconds using an LED light-curing unit. Then a putty tubing (5 X 2.5) was placed on top, and the composite resin was packed in 3 increments up to 5mm thick layer, and each increment was light-cured for 20 seconds.
Group 1B (n=30):
After adhesive light-curing:
Group 1C (n=30):
After adhesive light-curing:
Group 2B (n=30):
After adhesive light-curing:
Group 2C (n=30):
After adhesive light-curing:
The mode of failure of surfaces was then examined by stereomicroscope (MBC-10, SF-100b, Lomo, Russia) at 35 magnifications to determine the failure mode.
Microscopic observations revealed that the failures were mostly adhesive (Type I) at the tooth/cement interface, mostly cement (more than 75%) remains on the prepared tooth (Cacciafesta, Sfondrini et al. 2004). The data was entered into a computer and analyzed using statistical package for Social Science, (IBM-SPSS Inc., Chicago, IL, USA, Version 20) Description statistics (mean ± SD) was pertained to present the overview of the findings.Three-way analysis of variance (ANOVA) was pertained followed by a Scheffe Post-Hoc test to make a comparison among the groups. Statistical significance was set at P ≤ 0.05.
Results:
The mean difference of shear bond strength (MPa) in both total-etch and self-etch between adhesive and control groups were statistically significant (p<0.05). The highest shear bond strength was in the total-etch control group (13.2±0.08573) and the least was the self-etch control group (12.354±.28166), while the highest shear bond strength was in the self-etch adhesive group (9.9240±2.19319) and the least in the total-etch experimental group (7.9725±1.30558) (Figure 3).
The shear bond strength of self-etch group with air decontamination group showed the highest mean of 11.4018, and the shear bond strength of total-etch group with air decontamination group showed the least mean of 7.3295. While, the shear bond strength of self-etch group with water decontamination group showed the highest mean of 9.2743, and the shear bond strength of total-etch group with water decontamination group showed the least mean of 7.7372. Moreover, the shear bond strength of self-etch group with NaOCl decontamination group showed the highest mean of 9.0958, and the shear bond strength of total-etch group with NaOCl decontamination group showed the least mean of 8.8507.
The shear bond strength between blood contamination group and air decontamination group showed the highest mean of 9.9383, while the shear bond strength between haemostatic contamination and air decontamination group showed the least mean of 8.793.
Moreover, the shear bond strength between blood contamination decontamination and water showed the highest mean of 8.6276, while the shear bond strength between haemostatic contamination group and water decontamination group showed the least mean of 8.3839.
Furthermore, the shear bond strength between haemostatic contamination group and NaOCl decontamination group showed the highest mean of 11.1544, while the shear bond strength between blood contamination group and NaOCl decontamination group showed least mean of 6.7921.
In blood (D2), the mean difference in the shear bond strength (MPa) between air, water and NaOCl (D3) was highest between air and NaOCl (D3) and least between water and NaOCl (D3). However, the difference was statistically significant only between air and NaOCl (D3) (p<0.05) as shown in (Table 1).
In haemostatic(D2), the mean difference in the shear bond strength (MPa) between air, water, and NaOCl (D3) was highest between NaOCl and air (D3) and least between water and air (D3). Moreover, the mean difference between air, water, and NaOCl (D3) were all statistically significant (p<0.05) shown in (Table 2).
In blood (D2), the mean difference in the shear bond strength (MPa) between air, water, and NaOCl (D3) was highest between air and NaOCl (D3) and least between air and water (D3). The mean difference between air, water, and NaOCl (D3) were all statistically significant (p<005) (Table 3).
In haemostatic (D2), the mean difference in the shear bond strength (MPa) between air, water, and NaOCl (D3) was highest between NaOCl and water (D3) and least between NaOCl and air (D3). Also, the mean difference between air, water, and NaOCl (D3) were all statistically significant (p<0.05) (Table 4).
Descriptive analysis (Table 5) shows the mean, standard error of the mean, and 95% confidence intervals for the total-etch and self-etch (D1). Three-way analysis of variance (3-way ANOVA) shows a statistically significant difference in the mean shear bond strength (MPa) and interaction effect (p<0.05).
The mean (±SD) shear bond strength (MPa) of blood air self-etch (11.378±.86987) was highest in comparison to blood air total-etch (8.5018±.82040) (p<0.05). While the mean (±SD) shear bond strength (MPa) of blood water self-etch (9.2761±.92074) was highest in comparison to blood water total-etch (7.9792±.63334) (p<0.005).
The mean (±SD) shear bond strength (MPa) of blood NaOCl total-etch (7.6239±.75442) was highest in comparison to blood NaOCl self-etch (5.9603±.36473) (p<0.05). Moreover, the mean (±SD) shear bond strength (MPa) was highest in blood air self-etch (11.3748±.86987) in comparison to haemostatic air total-etch (6.1572±.29747) (p<0.05).
The mean (±SD) shear bond strength (MPa) was highest in blood water self-etch (9.276±.92074) in comparison to haemostatic water total-etch (7.4952±.23254) (p<0.000). Furthermore, the mean (±SD) shear bond strength (MPa) was highest in haemostatic NaOCl total-etch (10.0776±.32151) in comparison to blood NaOCl self-etch (5.9603 ±.36473) (p<0.000).
Differences in bond strength between total-etch and self-etch (D1) when contaminated with blood and haemostatic agent (D2) was highest in self-etch blood (8.8704±2.38304) in comparison to total-etch blood (8.0349±.80281), and highest in self-etch haemostatic (10.9776±1.34556) in comparison with total-etch haemostatic (7.9100±1.67788). Moreover, the differences were statistically significant (p<0.05) (Table 6).
Discussion:
Isolation of the working field is essential with any bonding procedure. Good isolation may be achieved with the use of a rubber dam. Unfortunately, it is not possible to use rubber dam in all clinical cases amongst general practitioners due to problematic and time-consuming, and when using cotton rolls during the bonding procedures, some kind of contamination may happen 2, 12
Blood contamination is a major clinical problem during restorative dental treatment 7. Kaneshima et al. (2000) stated that the effects of blood contamination on bond strength of adhesive resin to dentin may vary greatly depending on the adherent surface conditions. According to basic concepts of adhesion, the closer the contact between the adhesive and the adherent, the stronger is their junction 13.
Therefore, the contaminated layer may become a strong mechanical inhibitor of adhesion, preventing both adhesive system infiltration and polymerization, thus adversely affecting its bonding with the resin cement 8.
A mild self-etch adhesive is currently recommended for adhesion to dentin 14.
In the present study, contamination by blood decontaminated by air drying caused a decrease in bond strength between the adhesive and dentin, which is in agreement with previously published studies by Abdalla and Davidson 7. This situation might have left more blood contaminants on the dentin surface and also caused collagen fibrils to collapse, preventing adhesive monomers to infiltrate 15.
In this study, blood decontaminated by water caused bond strength reduction, this was in agreement with the studies done by Eiriksson 16, which evaluated the effects of blood contamination on resin-resin interfaces showing that blood protein components were not able to be completely rinsed away by a water spray, lowering the surface energy of the cured composite 7. Remnants of blood protein or excess water, which were not completely removed, could have impaired adhesion between the layers of adhesive and composite. Also, water rinsing could have disrupted the oxygen-inhibited and unpolymerized layer 17.
In this study, blood contamination decontaminated by NaOCl had the least bond strength to dentin, which was in agreement with the studies done by Prati, Chersoni 18, 19. NaOCl, being an oxidizing agent, has a negative effect on polymerization of dentin adhesives 20 Pyridinone cross-links occurring in collagen fibers get disrupted by NaOCl 21 and form chloramines- and protein-derived reactive free radicals 22 that interrupt with the chain propagation and do not allow complete polymerization to occur.
In this study haemostatic agent decontaminated by NaOCl in the self-etch adhesive system, showed the highest shear bond strength followed by air-dried and water respectively. This was in agreement with studies done by Prati et al 18. NaOCl increases the porosity of intact dentine by the removal of organic components of the demineralized collagen matrix 23, which will enhance adhesion to dentin by rendering the bonding substrate more susceptible to resin infiltration 24
In the present study, haemostatic agent decontaminated by NaOCl in the total-etch adhesive system showed the highest shear bond strength followed by water then air-dried. This is concerning the studies done by Prati et al 18, when re-etching is followed by NaOCl treatment, high bond strength can be achieved via reversed hybrid layer formation, a proposed new mechanism of micromechanical resin retention 18, 25.
In the present study, the haemostatic agent decontaminated by water in the self-etch adhesive system showed the least shear bond strength. This is in accordance to the previous study done by Kuphasuk et al 26 which found out that a greater amount of aluminium remained on the dentin surface than when the surface was treated with phosphoric acid when the dentin surface was treated by rinsing with water only 26. The remnants of the hemostatic agent on the dentin surface may result to a decrease in bond strength.
In the present study, it was found that the haemostatic agent significantly reduced the bond strength of both self-etch and total-etch adhesive to dentin. This is consistent with previous studies which showed that Aluminum chloride and Ferric sulphate dentin contamination can significantly lower the bond strength of self-etch adhesive compared to normal dentin 27, 28. What could interfere with the chemical bond and micromechanical retention of the self-adhesive resin cement to result to lower bond strengths is any remaining particles from hemostatic agents 26.
When contamination occurred following light curing of the adhesive, there is a statistically significant decrease in shear bond strength. This was in agreement with the results of the study done by Fritzl et al 28 by using one bottle adhesive systems which concluded that any contamination of the already cured adhesive layer seriously compromised the bond strength. Despite many differences in material properties, all the adhesives used in this study showed significant low bond strength after blood and haemostatic agent contamination.
The results from the present study indicated that when the dentin surface was contaminated after application of the adhesive system it influence the bond strength to dentin.
The results of this study reveal that the self-etch adhesive systems showed better bond strengths than the total-etch systems, in all the situations where both contaminants were used.
However, in the present study the adhesive failure occurs, might be due to contamination remnant on the dentin surface, thus interfering with the formation of a hybrid layer or inhibiting the binding of an adhesive system to resin cement.
Therefore, the most important factor for ensuring optimal bonding is to avoid contamination.
The results of the study demonstrated that both contaminants decreased the shear bond strength of the newer self-etch and total-etch adhesive systems to dentin. Thus, we have to reject the null hypothesis that blood and haemostatic agent contaminations do not affect the shear bond strength of the newer self-etch and total-etch adhesive systems to dentine.
References
Corresponding Author
Dr. Shibu Thomas Mathew
Assistant Professor
Riyadh Elm University, An Namudhajiyah, Riyadh 12734, Saudi Arabia
Phone no. +966534014050
Email: smathew @ riyadh.edu.sa
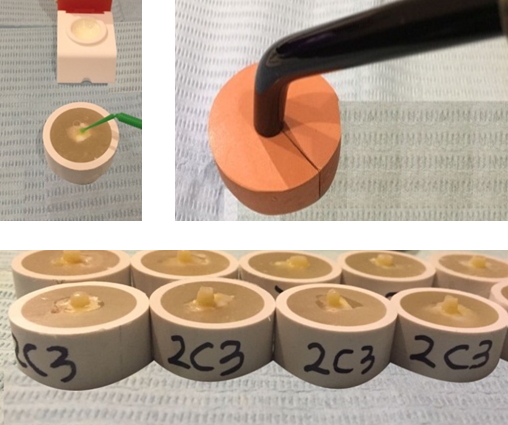
Figure 1. Adhesive application, light curing and composite resin placed on buccal surface.
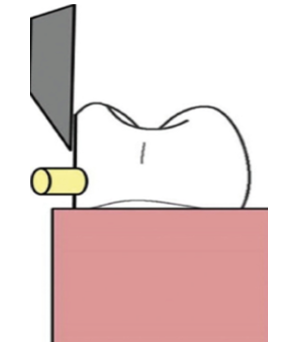
Figure 2. Schematic representation of the sample for testing.
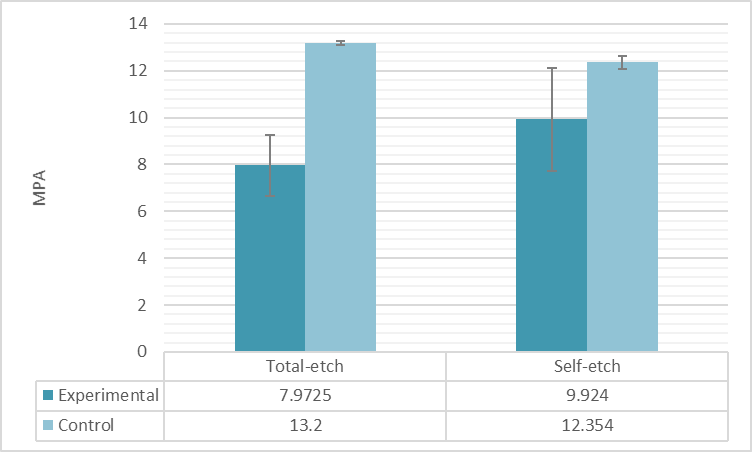
Figure 3. Mean and standard deviations (MPa) of the shear bond strength of adhesive and control groups.
Table 1. Total-etch group contaminated with blood.
|
Decontamination Group (D3) |
Mean Difference |
Std. Error |
Sig. |
95% Confidence Interval |
||
|
Lower Bound |
Upper Bound |
|||||
|
Air |
Water |
.5227 |
.33099 |
.303 |
-.3346 |
1.3799 |
|
|
NaOCl |
.8780* |
.33099 |
.044 |
.0207 |
1.7352 |
|
Water |
Air |
-.5227 |
.33099 |
.303 |
-1.3799 |
.3346 |
|
|
NaOCl |
.3553 |
.33099 |
.569 |
-.5020 |
1.2126 |
|
NaOCl |
Air |
-.8780* |
.33099 |
.044 |
-1.7352 |
-.0207 |
|
|
Water |
-.3553 |
.33099 |
.569 |
-1.2126 |
.5020 |
Table 2. Total-etch group contaminated with a haemostatic agent
|
Decontamination Group (D3) |
Mean Difference |
Std. Error |
Sig. |
95% Confidence Interval |
|||
|
Lower Bound |
Upper Bound |
||||||
|
|
Air |
Water |
-1.3381* |
.12805 |
.000 |
-1.6697 |
-1.0064 |
|
|
NaOCl |
-3.9204* |
.12805 |
.000 |
-4.2521 |
-3.5888 |
|
|
Water |
Air |
1.3381* |
.12805 |
.000 |
1.0064 |
1.6697 |
|
|
|
NaOCl |
-2.5824* |
.12805 |
.000 |
-2.9140 |
-2.2508 |
|
|
NaOCl |
Air |
3.9204* |
.12805 |
.000 |
3.5888 |
4.2521 |
|
|
|
Water |
2.5824* |
.12805 |
.000 |
2.2508 |
2.9140 |
|
Table 3. Self-etch group contaminated with blood
|
Decontamination Group (D3) |
Mean Difference |
Std. Error |
Sig. |
95% Confidence Interval |
|||
|
Lower Bound |
Upper Bound |
||||||
|
|
Air |
Water |
2.0987* |
.34034 |
.000 |
1.2173 |
2.9802 |
|
|
Naocl |
5.4145* |
.34034 |
.000 |
4.5330 |
6.2960 |
|
|
Water |
Air |
-2.0987* |
.34034 |
.000 |
-2.9802 |
-1.2173 |
|
|
|
Naocl |
3.3157* |
.34034 |
.000 |
2.4342 |
4.1972 |
|
|
Naocl |
Air |
-5.4145* |
.34034 |
.000 |
-6.2960 |
-4.5330 |
|
|
|
Water |
-3.3157* |
.34034 |
.000 |
-4.1972 |
-2.4342 |
|
Table 4. Self-etch group contaminated with haemostatic
|
Decontamination Group (D3) |
Mean Difference |
Std. Error |
Sig. |
95% Confidence Interval |
|||
|
Lower Bound |
Upper Bound |
||||||
|
|
Air |
Water |
2.1563* |
.20510 |
.000 |
1.6251 |
2.6876 |
|
|
Naocl |
-.8024* |
.20510 |
.002 |
-1.3336 |
-.2712 |
|
|
Water |
Air |
-2.1563* |
.20510 |
.000 |
-2.6876 |
-1.6251 |
|
|
|
Naocl |
-2.9587* |
.20510 |
.000 |
-3.4900 |
-2.4275 |
|
|
Naocl |
Air |
.8024* |
.20510 |
.002 |
.2712 |
1.3336 |
|
|
|
Water |
2.9587* |
.20510 |
.000 |
2.4275 |
3.4900 |
|
Table 5. Descriptive analysis of adhesive groups and their mean shear bond strength
|
D1 |
D2 |
D3 |
Mean |
Std. Error |
95% Confidence Interval |
|
|
Lower Bound |
Upper Bound |
|||||
|
Total-etch |
Blood |
Air |
8.502 |
.188 |
8.128 |
8.875 |
|
|
|
Water |
7.979 |
.188 |
7.606 |
8.353 |
|
|
|
NaOCl |
7.624 |
.188 |
7.250 |
7.997 |
|
|
Haemostatic |
Air |
6.157 |
.188 |
5.784 |
6.531 |
|
|
|
Water |
7.495 |
.188 |
7.122 |
7.869 |
|
|
|
NaoCL |
10.078 |
.188 |
9.704 |
10.451 |
|
Self-etch |
Blood |
Air |
11.375 |
.188 |
11.001 |
11.748 |
|
|
|
Water |
9.276 |
.188 |
8.903 |
9.649 |
|
|
|
NaOCl |
5.960 |
.188 |
5.587 |
6.334 |
|
|
Haemostatic |
Air |
11.429 |
.188 |
11.056 |
11.802 |
|
|
|
Water |
9.273 |
.188 |
8.899 |
9.646 |
|
|
|
NaOCl |
12.231 |
.188 |
11.858 |
12.605 |
Table 6. Inter-group comparison of the mean shear bond strength between contamination and decontamination groups
|
D2 D3 |
|
Mean |
Mean difference |
p-value |
|
Blood Air |
Self-etch |
11.378 |
2.8762* |
0.000 |
|
|
Total-etch |
8.5018 |
|
|
|
Blood Water |
Self-etch |
9.2761 |
1.2969* |
0.005 |
|
|
Total-etch |
7.9792 |
|
|
|
Blood NaOCl |
Total-etch |
7.6239 |
1.6636* |
0.000 |
|
|
Self-etch |
5.9603 |
|
|
|
Air Blood |
Self-etch |
11.3748 |
5.2176* |
0.000 |
|
Haemostatic |
Total-etch |
6.1572 |
|
|
|
Water Blood |
Self-etch |
9.2761 |
1.7809* |
0.000 |
|
Haemostatic |
Total-etch |
7.4952 |
|
|
|
NaOCl Haemostatic |
Total-etch |
10.0776 |
4.1173* |
0.000 |
|
Blood |
Self-etch |
5.9603 |
|
|
* indicates a statistically significant difference (p≤0.05)