|
Original Study |
Biocompatibility Of Calcium Silicate-Based Cement Incorporated With Silver Or Gold Nanoparticles - An In Vitro Study
Teena Sheethal Dsouza1*, Aditya Shetty2, Mithra N. Hegde3, Jenitta Emima Packayam4, Vishakh Radhakrishna5, Ashma Dorothy Monteiro6
1 Senior lecturer, Department of Conservative Dentistry & Endodontics, A.B.Shetty Memorial Institute of Dental Sciences, Nitte-Deemed to be University, India.
2Additional Professor, Department of Conservative Dentistry & Endodontics, A.B.Shetty Memorial Institute of Dental Sciences, Nitte-Deemed to be Universit, Indiay.
3 Vice Dean & Head of the Department, Department of Conservative Dentistry & Endodontics, A.B.Shetty Memorial Institute of Dental Sciences, Nitte-Deemed to be University, India.
4 Associate Professor, Department of Biotechnology, Alva’s College, Moodbidre, India.
5 Central Research Laboratory, K.S.Hegde Medical Academy, Nitte-Deemed to be University, India.
6 Assistant Professor (Selection Grade), Department of Data Science, Prasanna School of Public Health (PSPH), MAHE, Manipal, India.
|
|
Introduction
The success rate of conventional root canal treatment (RCT) has been reported in previous literature nearing to 95%. However, when the RCT fails, the first choice of treatment is non-surgical retreatment. When non-surgical retreatment is unwise in certain situations, surgical treatment is advised.1 A retro filling material following the surgical procedure seals the communication gap between the root canal and periapical tissues.2 An arduous search for an ideal retro filling material has led to the evolution of calcium silicate-based cement. The development of Mineral Trioxide Aggregate (MTA) has been a boon to endodontics with the ability to fulfill most of the ideal requirements of retro filling material.3 However, its major drawbacks include an increased set time and a limited antibacterial activity. Various combinations of MTA with additives have been tried to improve its properties. Few other retro filling materials have also been formulated to overcome the limitations of MTA.4 Available scientific literature on calcium silicate cement have shed light on their advantages and use in various applications in restorative dentistry and endodontics with a yield of good results. Their property of ‘bioactivity’ by eliciting favorable responses when in contact with periodontal tissues has been evident in various studies.5 Although a plethora of calcium silicate cement has been available in the market, the enhancement in their antibacterial property has been of question. The advent of nanotechnology in dentistry has shown to improve the physicochemical, mechanical, and antibacterial properties of dental materials.6 Nanotechnology is a good tool for delivering low molecular weight drugs and macromolecules such as proteins or genes to cells and tissues.7 Nanotechnology is an extremely vital space of research in trendy science and technology8. Silver and gold nanoparticles have been particularly used to inhibit bacterial growth in oral and endodontic applications.4,9-11 Nanoparticle (NP) delivery systems play important roles in increasing drug stability, prolonging the therapeutic period, and facilitating enteral and/or parenteral administration, which may minimize drug degradation and metabolism12. Nanoparticles are solid particles of a size of 10-100 mm13. However, the biologic response of these nanoparticles with the living dental tissues has to be evaluated.6 This study aimed to evaluate the biocompatibility of an experimental calcium silicate-based cement incorporated with either silver or gold nanoparticles.
Materials and Method:
Preparation of the experimental calcium silicate-based powder was done from pure oxides of powder and weighed on an analytical balance and mixed manually in a porcelain mortar until a uniform mixture was obtained. The experimental liquid component consisted of 10% calcium chloride. Synthesis of gold nanoparticles was done using extra pure chloroauric acid (Loba Chemie Pvt Ltd). Characterization of the gold nanoparticles was done using UV Vis Spectrophotometer (SL 159, ELICO). The size of the silver nanoparticles obtained commercially (SRL Chemicals) was 20nm.
Human lymphocytes were used in this study which was obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA). The cells were grown in Dulbecco modified Eagle medium (DMEM), supplemented with 5% fetal bovine serum, 100UmL-1 penicillin, 100μL mL-1 streptomycin, and 2 mmol-1 L-glutamine, at 37 ̊ C in a humidified atmosphere of 95% air and 5% CO2. Cement pellet was prepared by mixing the powder with the liquid.
The human lymphocyte cells were seeded into 96 well plates (2.7x104 cells) and incubated for 24 hours to allow adhesion. Then, 9 pellets of each experimental group were placed into the culture wells. Cells were treated in 3 experimental groups. Group 4 is the control group where the cells were not exposed to any experimental materials.
Group 1: Mineral Trioxide Aggregate (ProRoot MTA, Dentsply) with 1wt% silver nanoparticles + distilled water
Group 2: Experimental cement with 1wt% gold nanoparticles + 10% calcium chloride
Group 3: Mineral Trioxide Aggregate + distilled water
Group 4: no treatment (control group)
The cement pellets were tested at 0 hours for freshly mixed pellets (D0), after 24 hours (D1), 48 hours (D2), and 72 hours (D3) incubation for cytotoxicity by MTT assay. Spectroscopic absorbance (optical density) was measured at 630nm using an ELISA microplate reader. The experiment was repeated in triplicate.
Statistical analysis:
The results were evaluated by repeated measures of analysis of variance (ANOVA). Differences were considered significant at p < 0.05.
Results:
|
Group |
time |
Mean |
Std. Dev |
Std. Error |
95% Confidence Interval |
|
|
Lower Bound |
Upper Bound |
|||||
|
1.0 |
0 hour |
.212 |
0.0164 |
.015 |
.181 |
.242 |
|
|
24 hours |
.191 |
0.0362 |
.028 |
.135 |
.247 |
|
|
48 hours |
.227 |
0.0346 |
.017 |
.193 |
.261 |
|
|
72 hours |
.226 |
0.0317 |
.017 |
.192 |
.260 |
|
2.0 |
0 hour |
.339 |
0.0252 |
.015 |
.309 |
.370 |
|
|
24 hours |
.421 |
0.0782 |
.028 |
.365 |
.477 |
|
|
48 hours |
.272 |
0.0337 |
.017 |
.237 |
.306 |
|
|
72 hours |
.346 |
0.0422 |
.017 |
.311 |
.380 |
|
3.0 |
0 hour |
.249 |
0.0546 |
.015 |
.218 |
.280 |
|
|
24 hours |
.241 |
0.1252 |
.028 |
.185 |
.297 |
|
|
48 hours |
.173 |
0.0596 |
.017 |
.139 |
.207 |
|
|
72 hours |
.286 |
0.0559 |
.017 |
.252 |
.321 |
|
4.0 |
0 hour |
.391 |
0.0660 |
.015 |
.361 |
.422 |
|
|
24 hours |
.391 |
0.0660 |
.028 |
.335 |
.447 |
|
|
48 hours |
.391 |
0.0660 |
.017 |
.357 |
.426 |
|
|
72 hours |
.391 |
0.0660 |
.017 |
.357 |
.426 |
Table 1: Descriptive statistics
Repeated Measures ANOVA
Repeated measures ANOVA suggests that the value of outcome in each group differ significantly over the period (p<0.001).
Mauchly test suggests the violation of the assumption of sphericity (p<0.001), hence we report the conclusions based on Greenhouse-Geisser and Huynh-Feldt correction Both Greenhouse-Geisser and Huynh-Feldt corrections suggest that there is a significant difference between the outcomes of the experiment over a while, between the groups (p<0.001).
To identify whether there is a significant difference in the outcome between any two-time points, a paired t-test with Holm’s adjustment was performed.
|
Pairwise Comparisons |
||||||
|
Measure: MEASURE_1 |
||||||
|
(I) Group |
(J) Group |
Mean Difference (I-J) |
Std. Error |
Pvalueb |
95% Confidence Interval for Differenceb |
|
|
|
|
|
|
|
Lower Bound |
Upper Bound |
|
1.0 |
2.0 |
-.130* |
.020 |
< 0.001 |
-.186 |
-.075 |
|
|
3.0 |
-.023 |
.020 |
1.000 |
-.078 |
.032 |
|
|
4.0 |
-.177* |
.020 |
< 0.001 |
-.232 |
-.122 |
|
2.0 |
3.0 |
.107* |
.020 |
< 0.001 |
.052 |
.162 |
|
|
4.0 |
-.047 |
.020 |
.136 |
-.102 |
.008 |
|
3.0 |
4.0 |
-.154* |
.020 |
< 0.001 |
-.209 |
-.099 |
|
Based on estimated marginal means |
||||||
|
*. The mean difference is significant at the .05 level. |
||||||
|
b. Adjustment for multiple comparisons: Bonferroni. |
||||||
Table 2: Pairwise comparisons
The outcome of the experiment at only 48 hours is significantly different from the outcome at 0, 24, and 72 hours (p<.05).
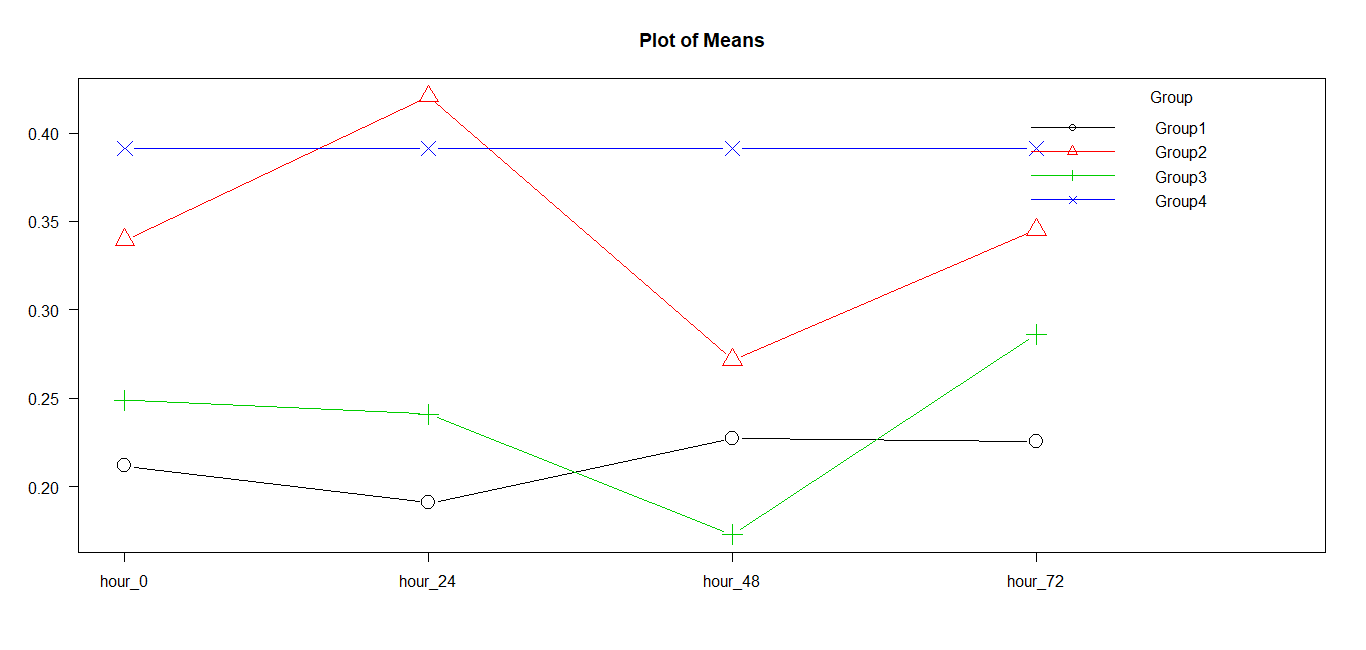
Graph 1:Plot of means for the groups.
There is no statistically significant difference between Group 2 and Group 4. Also, there is no statistically significant difference between Group 1 and Group 3.
Group 4 shows consistently higher values and Group 1 shows consistently lower values across all the time points. Group 2 and Group 3 are varying much with time.
Discussion:
The progression of the calcium silicate-based cements in endodontic and restorative applications has been quite remarkable. Their notable features of biocompatibility and bioactivity have made its use in endodontics utmost popular.5 Mineral Trioxide Aggregate is the first effectual calcium silicate-based material that has been introduced in endodontics.14 Alternative types of cement have been introduced in the market to better the existing cement. One such cement is Biodentine, that claims to have a shorter set time than MTA.15 The accelerated setting is most important because the risk of partial material loss and interface alteration during the final finishing procedures is minimized.16 However, the antibacterial and antifungal activities of these materials have been attributed only to their alkaline pH.17 Attempts have been made to improve the antimicrobial properties of calcium silicate-based cement. Silver nanoparticles addition to MTA has proven to inhibit E.Faecalis, P.aeruginosa, S.aureus, and C.albicans,3,4 A.actinomycetemcomitans, F.nucleatum, P.intermedia, and P.gingivalis10 in various studies. Recently, the use of gold nanoparticles in endodontic irrigation combined with lasers has been studied to reduce the microbial load inside the root canal.11 Their decreased toxicity combined with a wide array of possible chemical functionalizations has been considered, for utilization in a variety of applications.18 The cytotoxic reactions of calcium silicate-based cement combined with these nanoparticles need to be evaluated due to the proximity of these materials with the oral and periapical tissues.6 The cytotoxicity of the experimental groups was analyzed using the MTT assay. MTT depends upon the capacity of mitochondrial dehydrogenase enzyme to convert the water-soluble tetrazolium salt into dark blue formazan crystals. Cell viability depends on the quantity of formazan that is produced in the reaction.19 The cell lines that were used in this study were human lymphocytes. The advantage of using these cell lines is that they are karyotypically normal human cells and claim to be standard indicators for any systemic burden by exposure factors.20 Therefore, human lymphocytes were chosen in the present study.
Gold compounds are generally considered safe and have been in routine clinical use for many years, for example, in the treatment of rheumatoid arthritis.21 However, once reduced to the nanometer scale, particles are known to undergo profound changes in terms of their biochemical properties which necessitates renewed investigations into their cytotoxic profile.22 The results of our study showed that there was no statistically significant difference between the control group and group 2 (experimental cement with gold nanoparticles) in terms of cytotoxicity, suggesting that the overall biocompatibility of group 2 in the time was better than group 1 (MTA with silver nanoparticles) and group 3 (MTA with distilled water). The cytotoxic behavior of Groups 1 and 3 was similar. This leads to an indication that gold nanoparticles were relatively less cytotoxic when compared to silver nanoparticles. There are several studies in the literature that have proved the enhanced antimicrobial activity with the addition of silver nanoparticles to dental restorative materials. Most of these studies indicated that the nanosilver incorporated dental materials were relatively biocompatible.9, 23, 24 Currently, no studies are comparing the biocompatibility of silver and gold nanoparticles when combined with calcium silicate-based cement. Further studies are being carried out to determine the antibacterial activity.
Conclusion
Within the limitations of this study, it was concluded that the biocompatibility of the experimental calcium silicate-based cement was significantly improved when combined with gold nanoparticles. However, there was no difference between the nanosilver incorporated MTA and the gold standard MTA. Thus, silver and gold nanoparticles could be used as suitable additives to be incorporated into the experimental calcium silicate-based cement to improve the physical and mechanical properties.
References
Corresponding Author
Dr. Teena Sheethal Dsouza
Senior lecturer
Department of Conservative Dentistry & Endodontics
A.B.Shetty Memorial Institute of Dental Sciences
Nitte-Deemed to be University.
Email: tinsha_7 @ hotmail.com