ANALYSIS OF TRIGEMINAL NEURALGIA ASSOCIATED WITH NEUROVASCULAR COMPRESSION UTILIZING MRI: A SYSTEMATIC REVIEW AND META-ANALYSIS
Zygimantas Petronis1*, Raimundas Golubevas1, Jan Pavel Rokicki1, Vesta Guzeviciene1, Dalius Sakavicius1, Algirdas Lukosiunas1
1Department of Maxillofacial Surgery, Lithuanian University of Health Sciences, Kaunas, Lithuania. [email protected]
https://doi.org/10.51847/rDJGquURHA
ABSTRACT
The complete mechanism of Trigeminal neuralgia (TN) development is still not fully understood, knowing that several different factors can cause this disease. Magnetic resonance imaging (MRI) is an auxiliary tool to determine the possible cause. This paper reviews the architecture of the trigeminal nerve that is important to TN, describes the imaging appearance of vascular compression of the trigeminal nerve in TN patients, and discusses the relationship between these findings and the causative TN branch. Following the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) criteria, this systematic review and meta-analysis was carried out. The query set that was utilised was (neurovascular conflict) AND (trigeminal nerve) AND (neuralgia). The databases Google Scholar, ScienceDirect, ResearchGate, and PubMed/MEDLINE were utilised. The primary database search yielded 3886 articles; after applying search criteria, 4 articles were selected for the study. The methodological indicator of non-randomized studies - MINORS was used. The most common branches involved in the TN were a combination of second and third branches (55.6%). The overall difference was statistically insignificant when comparing the second and third trigeminal nerve branches with the most common neurovascular conflict vessels. MRI is the most used diagnostic tool for evaluating NVC in TN. The analyzed studies present the most frequent involvement of vertebral artery or superior cerebellar artery vessels in the NVC. Further large sample size studies are needed to develop a map (algorithm) that can easily identify the contacting vessel in the presence of branch TN.
Key words: Trigeminal neuralgia, Neurovascular conflict, Magnetic resonance imaging, Pain, Diagnostic.
Introduction
Facial pain is a frequent occurrence with various mechanisms of its development. Trigeminal neuralgia (TN), or tic douloureux, is one of the most common causes of facial pain [1]. TN is neuropathic pain involving one or more branches of the trigeminal nerve (cranial nerve V) [2]. This disease is characterized by stereotypic attacks that can last from a second to several minutes and are manifested by a feeling of "shooting," "electric shock," and "provocative zones" [3]. In the United Kingdom, the disease has been found to be the most common among women of all ages, with the highest incidence occurring between the ages of 45 and 59 years [4]. V2 and V3 are the branches most commonly affected by TN, whereas branch V1 occurs in less than 5% of all TN cases [5]. It has been established that even 17 percent of patients receive hospital TN treatment [6]. The complete mechanism of TN development is still not fully understood, knowing that this disease can be caused by several different factors (Figure 1).
|
|
|
Figure 1. Trigeminal neuralgia diagnosis scheme according to different factors [7]. TN, trigeminal neuralgia; MRI, magnetic resonance imaging; NVC, neurovascular conflict |
Magnetic resonance imaging (MRI) is used as an auxiliary tool to determine the possible cause [8]. When analyzing the MRI, contact between the nerve and blood vessels can be detected (neurovascular conflict (NVC)). It is hypothesized that an artery or vein's neurovascular pressure on the trigeminal nerve can disrupt neuronal disruption, resulting in neuropathic pain. During pressure, the myelin sheath and neurons are damaged, and as altered impulses are transmitted, a person perceives it and understands it as pain [9]. In common cases, the MRI method is not used to confirm the diagnosis of TN but to make a surgical decision [7]. The superior cerebellar artery was mobilised in a patient with TN following the first microsurgical decompression of the trigeminal nerve, which resulted in long-term pain alleviation. For patients who do not respond to conventional therapy, this procedure provides good and safe results [10].
Reviewing the trigeminal nerve architecture pertinent to trigeminal neuralgia (TN) and describing the imaging look of vascular compression of the trigeminal nerve in TN patients, along with their correlation with the causative TN branch, are the goals of this article.
Materials and Methods
Protocol and registration
Following the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) criteria, this systematic review and meta-analysis was carried out [11]. Our study protocol complied with PRISMA guidelines and was filed under the registration number CRD42023334610 with the International Prospective Register of Systematic Reviews and Meta-analysis (PROSPERO).
Literature selection
Two investigators independently queried PubMed/MEDLINE, ScienceDirect, ResearchGate, and Google Scholar databases to identify and select original research. The investigators reviewed all titles and relevant abstracts. The following combination of search terms was used: ((neurovascular conflict) AND (trigeminal nerve)) AND (neuralgia). Only English-language publications published in the last five years, from December 2017 to December 2022, were included in the literature search. There were no restrictions on the search based on publication status or country.
Randomised controlled trials, comparative studies, split-mouth randomised studies with double blinds, and controlled clinical trials in adult patients that determined the relationship between vascular conflict and pain of the various trigeminal nerve branches for patients with TN were among the inclusion criteria. Only one age group was the subject of one article rejection. Other research was not included, including case studies, studies using animals, systematic reviews, and meta-analyses.
Data extraction and quality assessment
Data from all included studies were extracted using a standard expressing form, and the following summary information was gathered: first author's surname and initials, year of submission, study design, total number of included cases, mean age, assessment, and results (branches and vessels of each patient).
Statistical methods
SPSS version 27 was used to conduct the statistical analysis. For the meta-analysis, MedCalc (Statistical Software Version 17.1) was employed. Because of the significant clinical heterogeneity across the included studies, a random effect model was employed for analysis. To assess statistical heterogeneity, the chi-square and I-square tests were employed. 25%, 50%, and 75% of the I2 values, respectively, showed low, moderate, and high heterogeneity. P values less than.05. was the threshold for statistical significance. The mean difference (MD) and its associated 95% confidence interval (CI) were used to demonstrate the impact of the assessment.
Results and Discussion
Literature search
The primary database search yielded 3886 articles after applying the search criteria. The full texts of 24 articles were retrieved and reviewed, and 20 articles were excluded as they failed to meet the inclusion criteria. Studies in the review were included if individual patient data was shown. A total of 4 articles were selected for study (Figure 2).
|
|
|
Figure 2. Flow diagram of the literature search and selection criteria. |
Reviewed studies included sample sizes of 1 to 22 patients. All 4 studies were presented, where NVC was detected using MRI [12-15]. After analyzing the articles, it was noticed that the sample sizes of all the conducted studies were not large enough (Table 1).
Quality assessment (Risk of bias)
Table 1. Data of interest
|
No |
Article, years |
Number of patients |
Age of patients (years) |
Methods of Determining Neurovascular Conflict |
|
1 |
Wang et al. (2021) [12] |
8 |
59,4 (42–71) |
GE 1.5T MR scanner; a time-of-flight (TOF) sequence was used to capture the pictures (TE minimum full bandwidth, 25 kHz; field of view, 24 cm; slice thickness, 1.0 mm). |
|
2 |
Son et al. (2022) [13] |
1 |
68 (68) |
3.0T MR machine |
|
3 |
Liu et al. (2019) [14] |
22 |
63 (44-80) |
MR machine and computer tomography |
|
4 |
Moon et al. (2018) [15] |
14 |
49 (36–64) |
7.0T MR machine |
MR, Magnetic resonance
All articles were analyzed after assessing the probability of errors (Bias). The methodological indicator of non-randomized studies – MINORS [16] was used. Two reviewers carried out the Risk of bias assessment independently; a third reviewer mediated disagreement. All information is presented in Figure 3.
|
|
|
Figure 3. Evaluation of the probability of errors in the analyzed articles. |
Outcomes
Wang et al. [12] analyzed 8 patients in their study and found that the second and third branches were the most common in TN, and the most common vessel involved in NVC was the superior cerebellar artery. In the study by Son et al. [13], NVC was detected in one patient. In another study by Liu et al. [14], it was found that the dominant blood vessel in NVC or in combination with other vessels was the vertebral artery (17), another blood vessel related to NVC was the superior cerebellar artery (12), veins (1), and anterior inferior cerebellar artery (6). Interestingly, after applying a special biomedical glue technique, as many as 20 out of 22 patients recovered (91%). Moon et al. [15] found that the most common blood vessel causing NVC was the superior cerebellar artery (11/14) (Table 2).
Table 2. The results of trigeminal nerve neuralgia studies, which were used in this review [12-15]
|
Article |
No. |
Sex |
Age |
Side |
Pain region |
NVC vessel |
|
Wang B et al. (2021) [12] |
1 |
M |
65 |
L |
V 2+3 |
VA |
|
2 |
M |
55 |
R |
V 2+3 |
SCA, SPV |
|
|
3 |
F |
71 |
R |
V 2+3 |
SCA |
|
|
4 |
F |
43 |
L |
V 3 |
SCA |
|
|
5 |
F |
63 |
R |
V 2+3 |
AICA |
|
|
6 |
M |
53 |
L |
V 1+2+3 |
SCA |
|
|
7 |
M |
68 |
R |
V 2+3 |
VA, SCA |
|
|
8 |
F |
58 |
R |
V 2+3 |
AICA |
|
|
Son B et al. (2022) [13] |
1 |
F |
68 |
R |
V 3 |
AICA |
|
Liu J et al. (2019) [14] |
1 |
M |
60 |
L |
V 3 |
VA,SCA |
|
2 |
D |
74 |
L |
V 2+3 |
VA,SCA |
|
|
3 |
M |
44 |
L |
V 2+3 |
VA |
|
|
4 |
M |
68 |
R |
V 2+3 |
BA,AICA |
|
|
5 |
F |
80 |
L |
V 2+3 |
VA,SCA |
|
|
6 |
M |
78 |
L |
V 2+3 |
BA, SCA |
|
|
7 |
F |
74 |
R |
V 2+3 |
BA |
|
|
8 |
M |
64 |
L |
V 2+3 |
VA, vein |
|
|
9 |
F |
50 |
L |
V 2 |
BA |
|
|
10 |
F |
62 |
L |
V 2+3 |
VA, SCA |
|
|
11 |
M |
75 |
R |
V 2+3 |
VA, SCA |
|
|
12 |
F |
67 |
R |
V 2+3 |
VA, SCA, AICA |
|
|
13 |
F |
66 |
L |
V 2+3 |
VA, AICA |
|
|
14 |
M |
64 |
L |
V 1+2+3 |
VA, AICA |
|
|
15 |
F |
56 |
L |
V 2+3 |
VA |
|
|
16 |
F |
55 |
R |
V 2+3 |
VA, AICA |
|
|
17 |
F |
61 |
L |
V 2+3 |
VA |
|
|
18 |
F |
57 |
L |
V 1+2+3 |
VA, SCA |
|
|
19 |
F |
65 |
R |
V 1+2+3 |
VA, SCA |
|
|
20 |
M |
51 |
R |
V 2+3 |
BA, SCA |
|
|
21 |
F |
45 |
L |
V 2 |
VA, SCA, AICA |
|
|
22 |
M |
64 |
R |
V 2 |
VA, SCA |
|
|
Moon H et al. (2018) [15] |
1 |
F |
50 |
L |
V 2 |
SCA |
|
2 |
F |
53 |
R |
V 2 |
SCA |
|
|
3 |
M |
36 |
L |
V 3 |
SCA |
|
|
4 |
F |
59 |
L |
V 2 |
AICA |
|
|
5 |
F |
31 |
R |
V 1+2+3 |
SCA |
|
|
6 |
M |
43 |
R |
V 2 |
AICA |
|
|
7 |
F |
48 |
R |
V 2+3 |
SCA |
|
|
8 |
F |
46 |
L |
V 2+3 |
AICA |
|
|
9 |
F |
62 |
R |
V 2 |
SCA |
|
|
10 |
F |
62 |
L |
V 2 |
SCA |
|
|
11 |
M |
64 |
L |
V 2 |
SCA |
|
|
12 |
F |
60 |
L |
V 2+3 |
SCA |
|
|
13 |
M |
36 |
R |
V 2 |
SCA |
|
|
14 |
F |
40 |
L |
V 2+3 |
SCA |
F, female; M, male; L, left, R, right; VA, vertebral artery; SCA, superior cerebellar artery; SPV, superior petrosal vein; BA, basilar artery; AICA, anterior inferior cerebellar artery; V, trigeminal nerve
After analyzing all articles, it is noticeable that the most common branches involved in the TN combine the second and third branches (55.6%). This combination occurs when NVC is between the trigeminal nerve, vertebral artery, or superior cerebellar artery. The tendency is observed that second branch TN usually was caused by superior cerebellar artery NVC.
Meta‑analysis outcomes
All studies were used in the meta-analysis [12-15] (Figures 4 and 5). The overall difference was statistically insignificant when comparing the second and third trigeminal nerve branch with most common NVC vessels.
This study demonstrates the utility of high-resolution MRI in the evaluation of NVC. There has been no extensive scientific research in the world that would help fully analyze the NVC and create a map (algorithm) that would easily determine the contacting blood vessel in the presence of TN of a certain branch. It was noticed that larger-scale studies do not specify which branch of the trigeminal nerve contacts which blood vessel, making it difficult to obtain significant, larger-scale results in this article.
Some articles noted that all studies were conducted using MRI to diagnose NVC. However, the authors write just intraoperative findings, which cannot be used in this review [17, 18].
There remains a tendency that radiologists do not always detect NVC after an MRI. In a study conducted by Singhal and Danks [19], it was found that radiologists' assessment can be reliable in 88% of cases in the examination of arterial NVC. The latest data describes the method of multispiral computed angiography as an alternative to MRI for the determination of NVC [20] and the method of MRI angiography [21].
It has been found that when patients present with "classic" TN, MRI offers high sensitivity and specificity for detecting NVC. According to Spanish research, having a well-established NVC on an MRI is a reliable indicator of long-term pain alleviation following nerve decompression surgery [22].
Brinzeu et al. [23] observed that MRI has a sensitivity of 97% and a specificity of 50% for the detection of NVC. Especially, it has good specificity outcomes from different tesla variations from 1.5 to 4.0 – the higher the mark, the better the results. The determination of vessel type is described as having high reliability, but the reliability of the determination of NVC grade is poor or moderate. It is mentioned that when a patient makes a decision about nerve decompression, the degree of pressure is the most important criterion for predicting the probability of long-term pain relief after the operative period.
It has been reported in the literature that the main cause of TN is artery compression of the nerve [24]. Nevertheless, current studies indicate that venous involvement in TN is not prevalent [25, 26]. The research by Dumot et al. [27] and Wang et al. [28] indicates that about 10% of cases might be due to venous compression alone. There was no solitary venous compression in our investigation. Regarding its clinical characteristics and surgical success, TN venous NVC is less well characterised than arterial NVC [28].
Analyzing NVC and identifying the arterial or venous conflict is important, but the distribution of trigeminal neuralgic pain significantly differs depending on the location of neurovascular conflict around the trigeminal nerve root [29].
As a result, TN is linked to neurovascular compression and may cause atrophy of the trigeminal nerve. Trigeminal nerve atrophy has been shown to result from severe and chronic NVC, which also tends to cause demyelination and axonal loss of the afflicted nerve [15, 30].
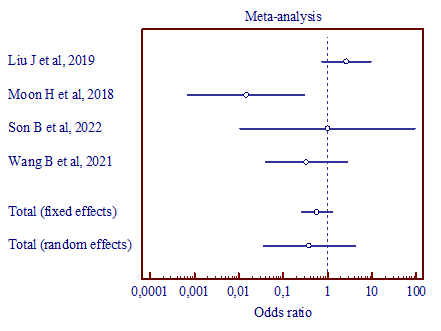 |
|
a) (MD, 0.386; 95% CI, 0.0357 to 4.172) |
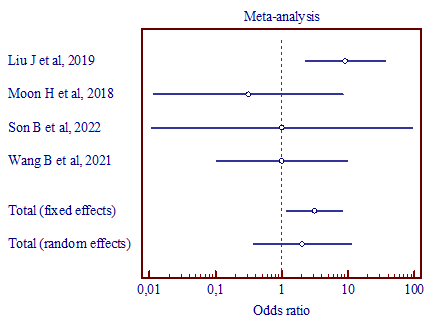 |
|
b) (MD, 2.035; 95% CI, 0.372 to 11.130) |
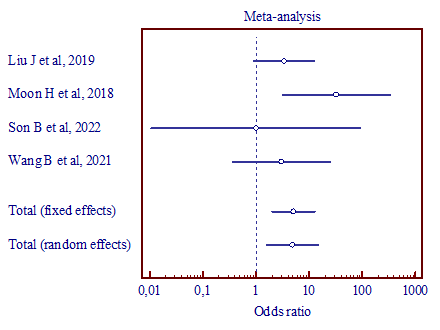 |
|
c) (MD, 4.824; 95% CI, 1.569 to 14.827) |
|
Figure 4. Results of the meta-analysis comparing the second trigeminal nerve branch with vertebral artery and superior cerebellar artery (a), vertebral artery and anterior inferior cerebellar artery (b), superior cerebellar artery and anterior inferior cerebellar artery (c). |
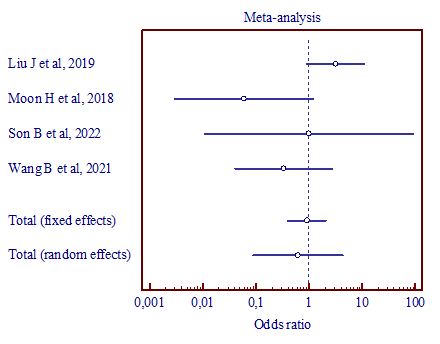 |
|
a) (MD, 0.613; 95% CI, 0.0874 to 4.297) |
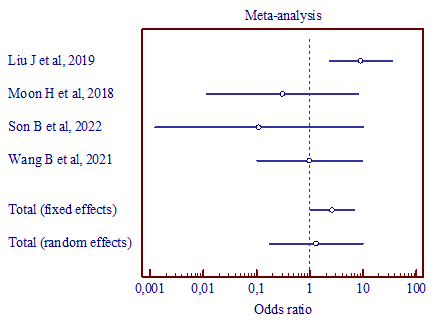 |
|
b) (MD, 1.327; 95% CI, 0.179 to 9.850) |
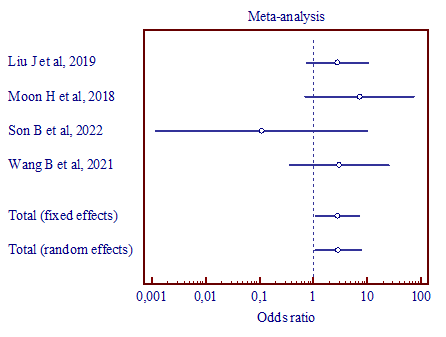 |
|
c) (MD, 2.916; 95% CI, 1.098 to 7.744) |
|
Figure 5. Results of the meta-analysis comparing the third trigeminal nerve branch with vertebral artery and superior cerebellar artery (a), vertebral artery and anterior inferior cerebellar artery (b), superior cerebellar artery and anterior inferior cerebellar artery (c). |
Conclusion
MRI is the most commonly used tool for evaluating NVC in TN. The analyzed studies present the most frequent involvement of vertebral artery or superior cerebellar artery vessels in NVC. The examined studies are small in scope. Therefore, we cannot present important, concrete conclusions. Further large sample size studies are needed to develop a map (algorithm) that can easily identify the contacting vessel in the presence of branch TN, and a unified diagnostic algorithm should be made with cooperation with radiologists, neuro, and maxillofacial/oral surgeons.
Acknowledgments: We would like to reaffirm that there are no known conflicts of interest related to this publication, nor has there been any substantial funding for this research that would have affected the results. We attest that each of the stated writers has read and authorised the work, and that no other individuals have met the requirements to be recognised as authors.
Conflict of interest: None
Financial support: None
Ethics statement: This study fulfills the ethical requirements of the Lithuanian University of Health Sciences.